Sperm cryopreservation is widely used in horse breeding to bank high-quality genetic material [1,2]. Nevertheless, cryopreservation causes a decrease in the number of motile spermatozoa and the fertilizing ability of sperm [1], as well as an increase in structural damage of germ cells [2]. During cryopreservation, spermatozoa are exposed to various factors damaging the cytoplasmic membrane, such as ice crystal formation, chemical toxicity, osmotic and oxidative stress [3].
The age of stallions is a key factor affecting cryopreservation success. Unlike other domestic animals, stallions have a longer reproductive lifespan and reach puberty later [4,5]. Studies indicate that sperm quality – including volume, concentration, total sperm count, and abnormalities – is lowest in stallions under 3 years and over 11 years of age. This age-related variation may stem from differences in daily sperm production, influenced by factors such as immature spermatogenesis in younger stallions, age-related testicular degeneration, and declining epididymal function in older animals [4]. Aurich et al. [6] observed a decline in the proportion of ejaculates suitable for cryopreservation in stallions older than 9 years. However, no significant differences in post-thaw sperm quality were found between stallions aged 2–4 years and those aged 5–9 years. Similarly, Dar et al. [5] reported that advancing age reduces total and progressive sperm motility, slows average sperm velocity, and increases reactive oxygen species (ROS) production. While existing research highlights the impact of age on sperm quality, the underlying molecular and biochemical mechanisms of stallion sperm aging remain poorly understood and warrant further investigation [7].
Redox regulation is central to sperm function. The oxidative stress observed in stallion sperm serves as a biomarker of systemic, age-related metabolic dysfunction, providing insights relevant to other mammalian species, including humans. Disruptions in redox homeostasis are a major factor in cryodamage following freeze-thaw cycles. Stallion spermatozoa are particularly prone to ROS generation because of their high mitochondrial activity, making them a valuable model for studying redox biology in sperm cells [8]. ROS play a dual role in sperm function: at controlled levels, they mediate essential redox signaling for capacitation and the acrosome reaction [9], but excessive ROS production leads to oxidative stress, loss of mitochondrial membrane potential (MMP), apoptosis, damaging lipids, proteins, and DNA [10]. To conduct a qualitative assessment of spermatozoa, it is necessary to evaluate not only progressive motility (PM) and viability but also mitochondrial function and oxidative stress markers. The assessment of redox status can be achieved by measuring ROS levels (e.g., via flow cytometry) and oxidative byproducts (such as the level of malondialdehyde [MDA] and oxidative modification of proteins [OMP]). In addition, both enzymatic (Superoxide dismutase [SOD], catalase, glutathione peroxidase, glutathione reductase) and non-enzymatic (Vitamins E and C, zinc, selenium) antioxidant defenses help regulate ROS levels and should be considered in redox metabolism analysis [11]. We propose that an integrated approach–evaluating sperm oxidative status, qualitative sperm characteristics, and mitochondrial function–will enable better regulation of ROS production in cryopreserved sperm across different age groups and improve the application of antioxidant therapy. This study aimed to determine the optimal stallion age for sperm cryopreservation by characterizing the relationship between age, redox homeostasis, and post-thaw sperm quality, thereby establishing a biochemical rationale for age-specific intervention protocols.
2. MATERIALS AND METHODS
2.1. Animals and Semen Collection
The study was conducted using cryopreserved sperm samples from 60 purebred Arabian stallions (aged 3–24 years) housed at the All-Russian Research Institute for Horse Breeding (Ryazan Region, Russia) and Tersk Stud Farm N169 (Stavropol Region, Russia). All procedures adhered to international and national ethical standards, including The Guide for the Care and Use of Laboratory Animals (8th edition, National Academies Press), CPCSEA guidelines, and The Russian Federation Law on Veterinary Medicine No. 4979-1 (May 14, 1993). The experimental protocol was approved by the Bioethics Commission of the All-Russian Research Institute for Horse Breeding (Protocol No. 2022/11/3).
All stallions included in the study underwent regular veterinary examinations, and only clinically healthy animals were selected. The stallions were housed in individual stalls within a stable, each equipped with automatic waterers. They received a minimum of four hours of daily exercise in paddocks. During the research period, the diet of the stallions consisted of grain-bean hay, oats, wheat bran, flaxseed cake, and carrots.
Samples were collected during the breeding season following a period of sexual rest. Each stallion provided three ejaculates at 48-h intervals, with only the third ejaculate used for research to ensure optimal sperm quality.
2.2. Sperm Freezing and Thawing
The cryopreservation of stallion semen was performed following the standard operating procedure established by the All-Russian Research Institute for Horse Breeding. This sperm cryopreservation technology has been successfully applied for several decades in horse breeding farms and equine reproductive centers across the Russian Federation. The semen was diluted using a lactose-chelated citrate-yolk extender, with glycerol serving as the cryoprotectant at a concentration of 3.5 mL/100 mL of extender (3.5%). After dilution and equilibration, the semen was packaged into labeled 20 mL aluminum tubes. Freezing was conducted in nitrogen vapor on a polyurethane stand, with the semen samples positioned 20 mm above the surface of the liquid nitrogen. The freezing process lasted 7 min, during which the temperature decreased from +4°C to −127°C over 420 s, at a cooling rate of 3.2°C/s. The frozen semen was stored in liquid nitrogen at −196°C until examination or artificial insemination of mares. For thawing, the samples were immersed in a water bath at +40°C for 90 s. This thawing protocol has been determined to be optimal for this cryopreservation method [12].
2.3. Total Motility (TM) and PM Assay
TM and PM was assessed after thawing using the Argus CASA system (ArgusSoft LTD., Saint Petersburg, Russia) and a Motic BA 410 microscope (Motic, Hong Kong, China) in a Mackler chamber at 37°C.
2.4. Determination of ROS, Apoptosis, and MMP by Imaging Flow Cytometry
Following thawing, semen samples were centrifuged twice (2000 rpm, 5 min) in warm Phosphate-Buffered Saline (PBS) containing 0.1% Bovine Serum Albumin to remove cryopreservation medium. ROS production was assessed using two fluorescent probes: DCFH-DA (2,7-dichlorofluorescin diacetate; Elabscience, China) – general intracellular ROS indicator (green fluorescence) and dihydroethidium (DHE) (Biotium, USA) – a superoxide-indicative probe (red fluorescence).
For DCFH-DA analysis, 1 × 106 spermatozoa in 100 µL PBS were incubated with 40 µM DCFH-DA (30 min, 37°C, dark). After washing (2000 rpm, 5 min), samples were counterstained with 1 μL PI (25 μg/mL). Flow cytometry analysis distinguished viable ROS-positive sperm (DCFH-DA+/PI-), viable ROS-negative sperm (DCFH-DA-/PI-).
To determine the superoxide anion, 1 × 106 spermatozoa were resuspended in a calcein AM solution with a final concentration of 0.01 µM (Elabscience, China), DHE was added at a final concentration of 2 µM, and incubated for 15 minutes at a temperature of 37°C. The proportions of live spermatozoa positive for DHE (DHE+/calcein AM+) and negative for DHE (DHE-/calcein AM+) were analyzed.
The Annexin V-FITC/PI Apoptosis Kit (Elabscience®, China) was utilized to assess alterations in the plasma membrane of stallion spermatozoa, specifically focusing on phosphatidylserine translocation. In this procedure, 1 × 106 spermatozoa were suspended in 200 µL of 1× Annexin V Binding Buffer and incubated with 4 µL of Annexin V-FITC, which had been diluted 2-fold in PBS, for 10 min in the dark at room temperature. Following this, 2 µL of propidium iodide (PI) solution (25 µg/mL) was introduced, and the samples were analyzed using flow cytometry. The percentage of spermatozoa that were positive for Annexin V (AnV+/PI− and AnV+/PI+) was interpreted as the proportion of spermatozoa exhibiting apoptotic-like characteristics.
To evaluate the MMP, a MMP assay kit (Elabscience®, China) was employed, following the manufacturer’s guidelines with some adjustments. The assay measures the uptake of the JC-1 dye, where a higher concentration of JC-1 aggregates correlates with a red fluorescent emission, in contrast to the green fluorescence emitted by JC-1 monomers. The results were expressed as a red-to-green fluorescence ratio. In addition, a sample treated with CCCP, an uncoupler of oxidative phosphorylation and tissue respiration, served as a negative control.
Cytometric studies were performed on a Cytek®Amnis®Flowsight device (Cytek Biosciences, USA) using a 488 nm blue laser with a power of 60 mW (ROS level) and 20 mW (apoptosis and MMP) at a medium flow rate using INSPIRE™ software. 10,000 events were analyzed using the IDEAS®6.3 software. To establish proper gating parameters and compensation settings, unstained, single-stained, and positive controls were used to differentiate between positive and negative events and define regions of interest.
2.5. Biochemical Analysis of Spermatozoa
Thawed ejaculates were centrifuged (6,000 rpm, 5 min) to remove the cryoprotectant diluent, followed by two washes in PBS under the same conditions. The resulting pellet was resuspended in bidistilled water containing 0.1% Triton X-100 and stored at −18°C until analysis.
Catalase activity was measured spectrophotometrically based on hydrogen peroxide’s reaction with molybdenum salts to form a stable colored complex [13]. Lysed sperm cells (0.1 mL) were mixed with 2 mL of 0.03% H2O2 solution. After a 10-min incubation, the reaction was stopped by adding 1 mL of 4% ammonium molybdate. Blanks were prepared using distilled water instead of the cell lysate. The extinction (absorbance) was measured at 410 nm using an SP- 2000 spectrophotometer (OKB Spektr LLC, Russia), with the sample probe compared against the blank probe. Catalase activity (A) was calculated using the formula: A = ((Eb-Es) • 3.1•106) /(v • t • ε • C), where: A =catalase activity (nmol/min*mg protein), Eb = extinction of the blank probe, Es = extinction of the sample probe, 3.1 = total volume (mL), v= sample volume (0.1 mL), t = incubation time (10 min), ε = extinction coefficient (22.2 × 10³ ml/mmol/cm), C = protein concentration (mg/ml), 106 = mmol → nmol conversion.
SOD activity was assessed photometrically by quantifying its inhibition of quercetin autooxidation [14]. The oxidation of quercetin in the presence of tetramethylethylenediamine (TEMED) at pH 10 is a free radical chain reaction involving superoxide and is therefore inhibited by SOD. The degree of inhibition of quercetin oxidation depended on the activity of SOD. The quantitative parameters of the reaction were determined on Stat Fax 4500+ (Awareness Technology Inc, USA) by measuring the optical density of the reaction mixture at a wavelength of 406 nm. The sample contained 0.5 mL of 0.2 M Bicarbonate buffer (pH = 10.0), 0.5 mL of TEMED working solution, 1.8 mL of distilled water, and 0.1 mL of sperm lysate. The blank and control each contained 0.5 mL of 0.2 M Bicarbonate buffer (pH = 10.0), 0.5 mL of TEMED working solution and 2.0 mL of distilled water for the blank sample (used to set 0 and prepare 1 for the batch) and 1.9 mL for the control sample (used to measure free autooxidation of quercetin in the absence of SOD and is prepared 1 per batch). The reaction is initiated by adding 0.1 mL of 0.5 mM quercetin solution to DMSO to the control and experimental samples, the contents are mixed, and the optical density against the blank sample is immediately recorded. After 10 min, the decrease in the optical density of the solution was measured. The calculation was carried out according to the formula: A = (ΔEc-ΔEs)/(a*V), where: A= is the activity of SOD, conventional units per mg of protein (c.u/mg of protein); ΔEc = decrease in the optical density of the control sample in 10 min; ΔEs = decrease in the optical density of the sample in 10 min; a = protein concentration in the sample in mg/mL; V = volume of sperm lysate in mL.
Lipid peroxidation (LPO) was evaluated through thiobarbituric acid-reactive substances (TBARS) using a commercial MDA assay kit (Elabscience®, China). When heated under acidic conditions, malondialdehyde (MDA) and other secondary LPO products react with TBA to form a pink-colored adduct. This chromogen exhibits maximum absorbance at 530–540 nm, with its optical density being directly proportional to the TBARS concentration in the sample.
Protein carbonylation was determined using the spectrophotometric Levine-Dubinina method [15] as described in [16]. This method quantifies protein carbonyl groups formed through oxidative damage by their reaction with 2,4-dinitrophenylhydrazine (2,4-DNPH) to form stable derivatives – aldehyde (ADNPH) and ketone (KDNPH) dinitrophenylhydrazone. Amino acid derivatives were measured at specific wavelengths: ADNPH (neutral) - 230, 254, 270, 280, 356 nm; KDNPH (neutral) – 363, 370 nm, ADNPH (basic)- 428, 430 nm; KDNPH (basic)- 434, 524, 530, 535 nm. Absorption spectra of OMP products were plotted using the obtained extinction values. Total OMP was calculated as the sum of areas under the curve for aldehyde carbonyl derivatives and ketone carbonyl derivatives according to the formulas as described in the author’s patent [17]. Results were expressed in conventional units per gram of protein (c.u./g protein).
Total protein concentration was quantified with the Bradford method (Coomassie-based assay kit, Servicebio, China). Activity of enzymes, OMP and MDA levels were described based on protein normalization.
2.6. Statistical Analysis
Sixty stallions were enrolled and stratified into three age groups–younger (3–5 years, n = 12), mature (6–15 years, n = 29), and older (16–24 years, n = 19). The exact sample size analysed for each parameter (biochemistry, CASA, flow cytometry) is stated in the figure and table legends (minimum n = 5, maximum n = 29). Sample size was determined a priori using G*Power 3.1 (one-way analysis of variance, α = 0.05, three groups). The effect size was taken from reports [18,19]. The selection of the minimum number of animals was carried out according to the “resource-equation” (E-rule) [20].
Data analysis was performed in GraphPad Prism 9 and Excel 2016 (StatSoft Inc.). Normality was assessed using the Shapiro–Wilk test; due to non-normal distributions, nonparametric Kruskal-Wallis tests were applied. Multiple comparisons were adjusted through the Benjamini–Krieger–Yekutieli method. Spearman’s correlation analysis was used to evaluate the relationship between two ranked variables. Results are reported as median (interquartile range), with P < 0.05 considered statistically significant.
3. RESULTS AND DISCUSSION
3.1. ROS Level, Apoptosis, and MMP
Cryopreservation leads to the formation of ROS in the cell. It is known that photophysical phenomena occur in the frozen aquatic system at −196, leading to the formation of free radicals and the rupture of macromolecules [21]. The most commonly used fluorescent probe for flow cytometry ROS assays is DCFH-DA and its derivatives. These probes offer several advantages, including ease of use, cell permeability, high sensitivity to cellular redox changes, and cost-effectiveness. To enhance accuracy, many researchers employ DCFH-DA in combination with PI, which helps minimize autofluorescence artifacts and restricts ROS measurement to viable cells, excluding compromised or dead cells from the analysis [22]. However, a major limitation of DCFH-DA is its lack of specificity, as it detects total intracellular ROS rather than individual reactive species. To address this, researchers have combined DCFH-DA with more specific probes, such as DHE [23]. Some authors think that DHE is not a specific fluorescent probe for superoxide detection and requires additional HPLC analyses [24]. However, it has been shown that a combination of DHE with calcein AM can be successfully used for superoxide radical detection in equine spermatozoa [25]. Building on these methodological advancements, we assessed ROS levels in spermatozoa using a dual-staining approach: DCFH-DA with PI to evaluate total ROS in sperm [Figure 1a] and DHE with calcein AM for superoxide-specific detection [Figure 1b].
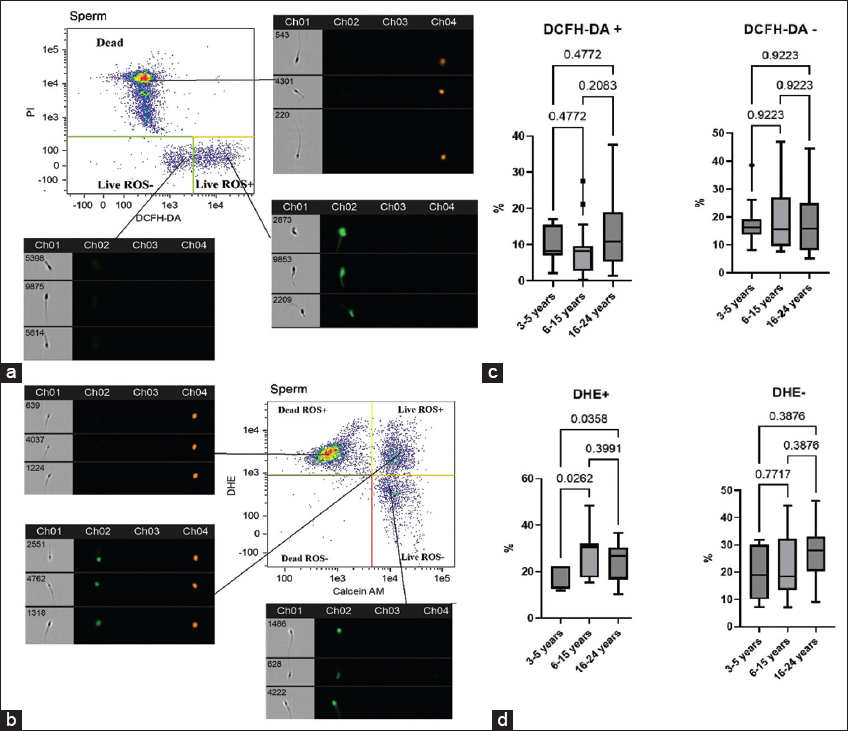 | Figure 1: Representative imaging flow cytometry results of ROS levels in cryopreserved spermatozoa. Sperm populations were initially gated based on morphological characteristics using an Area/Aspect ratio dot plot. Subsequent analysis involved creating fluorescence dot plots that simultaneously distinguished. (a) Viable (PI-) versus non-viable (PI+) spermatozoa in the red channel (Ch04), and ROS-positive (DCFH-DA+) versus ROS-negative (DCFH-DA-) spermatozoa in the green channel (Ch02). (b) Viable (Calcein AM+) versus non-viable (Calcein AM-) spermatozoa in the green channel (Ch02), and superoxide production (DHE+ [ROS-positive] vs. DHE- [ROS-negative]) in the red channel (Ch04). These populations were then categorized into four distinct quadrants based on their fluorescence profiles. (c) Proportion of live spermatozoa positive (DCFH-DA+/PI-) and negative (DCFH-DA-/PI-) for DCFH-DA, median (IQR) (3–5 years n = 12, 6–15 years n = 29, 16–24 years n = 19). (d) Proportion of live spermatozoa positive (DHE+/PI-) and negative (DHE-/PI-) for DHE. [Click here to view] |
Analysis of flow cytometry data did not reveal significant differences in the total level of ROS (DCFH-DA+/PI- and DCFH-DA-/PI-) in groups of stallions of different ages [Figure 1c]. At the same time, a significant difference in the level of superoxide was found. The proportion of viable, ROS-positive spermatozoa (DHE+/calcein AM+) was significantly lower in younger stallions compared to both mature and older stallions [Figure 1d]. These findings align with established literature demonstrating an age-dependent increase in seminal ROS levels [26].
There is evidence that cryopreservation leads to a short-term release of mitochondrial ROS, which initially causes capacitation in a subpopulation of spermatozoa; however, later osmotic stress of mitochondria leads to a decrease in mitochondrial function with a decrease in ATP levels, loss of MMP, and sperm motility [27]. In connection with the above, we studied the MMP in cryopreserved sperm [Figure 2a and b]. The group of stallions aged 16–24 years was characterized by a decrease in mitochondrial potential in thawed sperm. Significant differences were found in comparison with the group of mature stallions [Figure 2c].
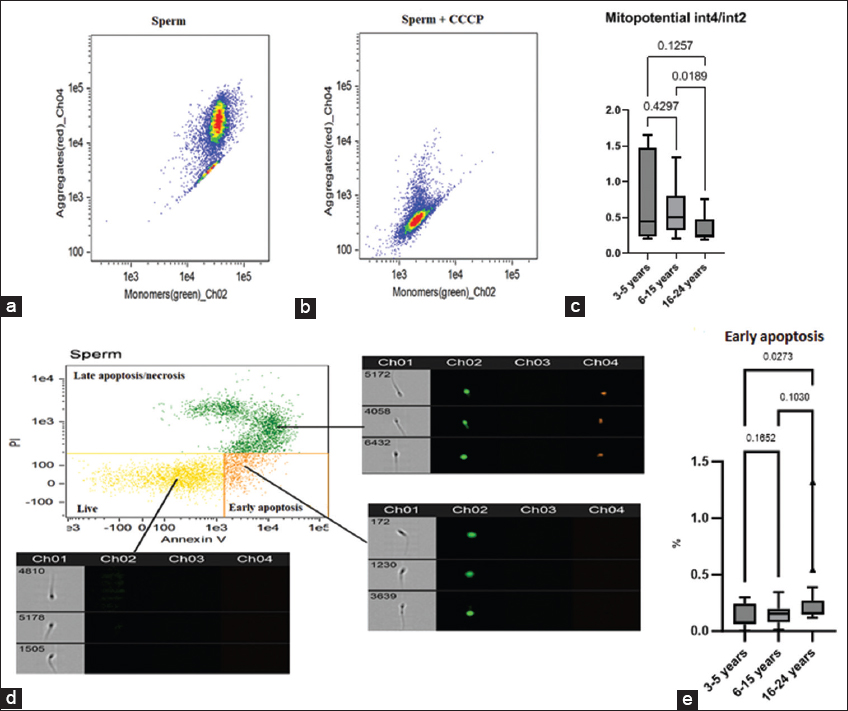 | Figure 2: Representative imaging flow cytometry results of mitochondrial membrane potential (MMP) and apoptosis in cryopreserved spermatozoa. Spermatozoa populations were initially gated based on morphological characteristics using an Area/Aspect ratio dot plot. (a) Example of MMP assessment with JC-1 in cryopreserved semen. The x axis (Ch02) reflects monomers fluorescence; the y axis (Ch04) reflects aggregates fluorescence. (b) Example of MMP assessment in cryopreserved semen with CCCP addition. (c) Results of red-to-green fluorescence ratio in cryopreserved semen of different age stallions, median (IQR) (3–5 years n = 8, 6–15 years n = 20, 16–24 years n = 15). (d) Apoptosis assessment. The x axis (Ch02) reflects annexin V–FITC fluorescence; the y axis (Ch04) reflects PI fluorescence. Subsequent analysis employed dual-fluorescence dot plots to simultaneously evaluate: late apoptosis/necrosis spermatozoa (Annexin V+/PI+ and Annexin V-/PI+), viable (Annexin V-/PI-) and spermatozoa in early apoptosis (Annexin V+/PI-). (e) Proportion of spermatozoa in early apoptosis, median (IQR) (Annexin V+/PI-) [Click here to view] |
Hyperproduction of ROS by mitochondria may be accompanied by the development of an internal apoptosis pathway with externalization of phosphatidylserine, activation of the caspase cascade, vacuolization of the cytoplasm, and oxidative damage to DNA. It is assumed that mature spermatozoa have a functioning phosphatidylinositol-3-kinase/protein kinase B signaling pathway, which acts by maintaining the pro-apoptotic protein Bcl-2-associated death promoter in a phosphorylated, anti-apoptotic state [28]. The translocation of phosphatidylserine to the outer leaflet of the plasma membrane is typically detected using Annexin V. In our experiments, we used Annexin V in combination with the dead cell dye PI [Figure 2d]. We found a significant increase in the percentage of spermatozoa in the early apoptosis stage in the group of older stallions compared with younger stallions [Figure 2e].
3.2. OMP
Sperm proteins are susceptible to redox-dependent modifications that can have dual effects depending on ROS levels. At moderate concentrations, ROS modulate signaling pathways critical for sperm physiology, either activating or inactivating them. However, excessive ROS can cause oxidative damage, impairing vital cellular functions. Some of these modifications are reversible, enabling precise regulation of redox signaling processes [29]. In contrast, protein carbonylation is an irreversible oxidative post-translational modification that introduces reactive carbonyl groups–such as aldehydes, ketones, or lactams–into proteins. These modifications progressively accumulate throughout an organism’s lifespan and serve as biomarkers for oxidative stress, often being quantified to assess cellular damage, aging, and age-related diseases [30-32].
A comparative analysis of oxidized protein byproducts in cryopreserved spermatozoa revealed that older stallions exhibited the highest OMP levels, surpassing those of younger and mature animals [Figure 3].
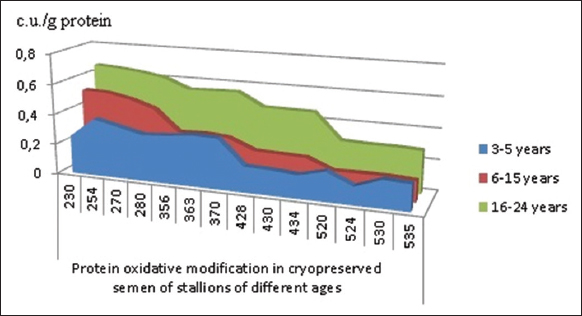 | Figure 3: Absorption spectrum of oxidative protein modifications in cryopreserved stallion sperm across different age groups (c.u./g protein), median. [Click here to view] |
A statistically significant increase in the total area under the absorption spectrum curve of oxidized protein modification products was observed across all studied carbonyl derivatives [Table 1]. This trend aligns with our previous findings in stallion seminal plasma, where older stallions exhibited higher OMP levels than younger stallions, primarily due to neutral ADNPH derivatives [33]. Similarly, peripheral blood mononuclear leukocytes from older stallions showed a significant rise in total protein carbonyl content. This increase was driven by elevated levels of neutral and basic carbonyl derivatives compared to younger and mature stallions [34].
Table 1: Absorption spectrum of oxidative protein modifications in cryopreserved stallion sperm across different age groups (c.u./g protein), median (IQR).
| Indicator | Age of stallions, years | ||
|---|---|---|---|
| (1) 3–5 n=7 | (2) 6–15 n=20 | (3) 16–24 n=17 | |
| SADNPH Neutral | 71.76 | 108.04 | 188.33 |
| SADNPH Basic | 25.36 | 36.52 | 86.36 |
| SKDNPH Neutral | 23.14 | 29.75 | 69.02 |
| SKDNPH Basic | 3.74 | 6.23 | 13.60 [8.55;42.13] |
| STOTAL | 124.00 | 167.87 | 356.62 |
3.3. MDA Level, Catalase, and SOD Activity
The lowest level of MDA was found in the group of younger stallions compared to the group of mature and older stallions [Figure 4a]. MDA is a secondary molecular product of LPO and a recognized biomarker of oxidative stress [35-37]. LPO in cryopreserved stallion sperm negatively affects fertility and viability, causing membrane damage and DNA fragmentation [38]. The high content of polyunsaturated fatty acids in the plasma membrane of equine sperm makes them susceptible to the effects of free radicals during the freezing and activation of LPO [8]. In the native spermatozoa of stallions with poor cryostability, the ratio of unsaturated fatty acids to saturated was twice as high as in stallions with good cryostability, indicating a positive effect of a high ratio of saturated fatty acids to unsaturated on the cryostability of equine spermatozoa [39]. The lipid composition of sperm membranes changes with age, which affects their resistance to cryopreservation and negatively affects the quality of cryopreservation [40]. It could be a possible mechanism explaining data obtained.
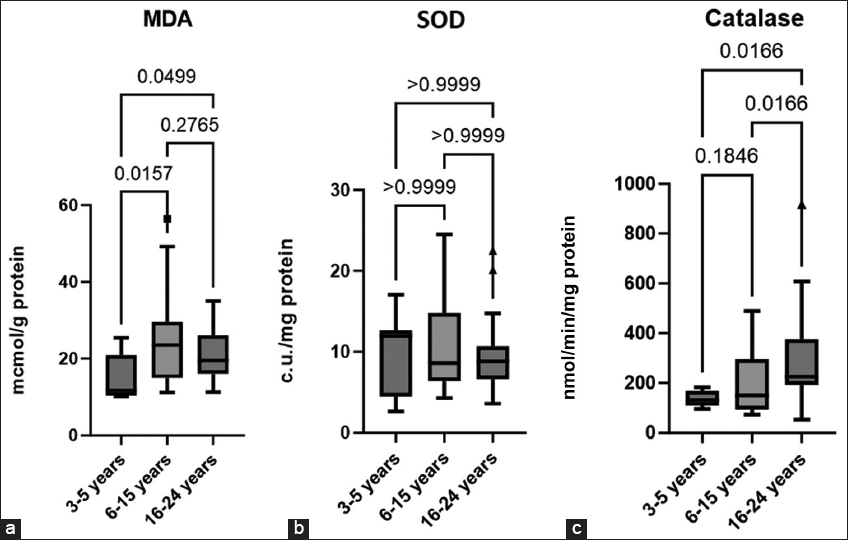 | Figure 4: Malondialdehyde concentration (a), Superoxide dismutase activity (b), catalase activity (c) in cryopreserved stallion sperm across different age groups, median (IQR) (3–5 years n = 9, 6–15 years n = 20, 16–24 years n = 16). [Click here to view] |
During cellular detoxification, the SOD-CAT-GPX catalytic triad plays a crucial role in protecting spermatozoa from excessive ROS by providing antioxidant defense [22]. However, cryopreservation has been shown to significantly reduce SOD (Cu-Zn) (SOD1) levels in stallion spermatozoa [41]. In our study, no significant differences in SOD activity were observed among the examined stallion groups [Figure 4b].
SOD converts superoxide (O2•−) into hydrogen peroxide (H2O2), which can then be neutralized to form water by several peroxidases, among which catalase, a heme-containing enzyme that reduces H2O2 to water and molecular oxygen (2 H2O2 →2 H2O+O2), plays an important role [35]. We have identified a trend in increasing catalase activity with age - the highest activity of the enzyme was found in the spermatozoa of older stallions, significant differences were obtained compared with younger and older stallions [Figure 4c]. Interesting data were obtained on mice knocked out by the SOD and catalase gene. Aged SOD−/−mice showed pronounced redox dysfunction, while wild-type and CAT−/− mice possessed compensatory mechanisms that partially attenuate oxidative stress and reduce age-related DNA damage in spermatozoa. The authors of the study concluded that SOD1, but not catalase, is crucial for maintaining germ cell quality during aging [42]. At the same time, it was shown that overexpression of catalase in mice protected against age-related decrease in sperm count, loss of germ cells, and Sertoli cells. In addition, the spermatocytes of elderly mice with catalase overexpression maintained low ROS levels and ensured their effective removal, and in spermatozoa, catalase overexpression reduced age-associated DNA damage [43].
According to some authors, catalase is contained in peroxisomes and the cytoplasm of cells, and spermatozoa, practically devoid of cytoplasmic components, can contain catalase only in small amounts [44]. According to some reports, the catalase test can serve as an indirect indicator of the presence of pus and blood, as well as bacterial contamination of semen [45]. However, recent literature data indicate species-specific adaptation to oxidative stress and emphasize the role of catalase as a key antioxidant in equine semen. According to the results of immunocytochemistry, catalase was detected in the head of equine spermatozoa, especially in the postacrosomal region. It was also shown in this study that catalase, rather than glutathione peroxidase, makes a more significant contribution to the neutralization of hydrogen peroxide in horse semen [46]. In our study, an increase in catalase activity in the group of older stallions could have occurred due to the strain on the antioxidant system, due to the tendency to increase ROS level [47].
3.4. PM in Cryopreserved Spermatozoa and its Relation to Indicators of Oxidative Stress
An analysis of the data on the progressive and general motility of spermatozoa after cryopreservation showed that the group of mature stallions has the highest level of PM compared to younger and older stallions [Figure 5a]. A similar trend is observed with respect to the total motility of spermatozoa after cryopreservation [Figure 5b].
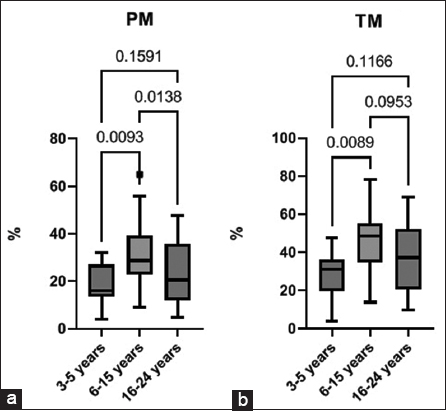 | Figure 5: Progressive motility (a) and total motility (b) in cryopreserved stallion sperm across different age groups, median (IQR) (3–5 years n = 12, 6–15 years n = 29, 16–24 years n = 19). [Click here to view] |
We also found a moderate negative correlation between the level of PM after cryopreservation and the level of aldehyde dinitrophenylhydrazones of a neutral nature (r = −0.323; P = 0.048). The data obtained indicate a connection between a decrease in the PM of spermatozoa and the intensification of oxidative stress.
Aging in stallions leads to the accumulation of oxidative damage in spermatozoa. When these cells undergo additional stress, such as cryopreservation, the imbalance between ROS production and antioxidant defenses becomes more pronounced [48]. In our study, the group of mature stallions has significantly higher superoxide levels than the group of younger stallions, and has no significant differences in ROS levels compared to the group of older stallions, as shown by DHE [Figure 1d]. Superoxide anion is formed in the mitochondrial respiratory chain during its active OXPHOS work to provide ATP to the sperm, and OXPHOS is the main way of obtaining energy in stallion sperm [49]. Apparently, in mature stallions, with normal antioxidant protection, this process does not entail the development of oxidative stress [48]. In these stallions, ROS levels are sufficient to support signaling without overwhelming antioxidant capacity, ensuring adequate ATP production and sustained motility.
However, in the spermatozoa of older stallions, the same level of ROS can become critical probably due to an imbalance in antioxidant system, which mirrors as a compensatory increase in catalase activity [Figure 4c]. Accumulation of ROS leads to a decrease in MMP [Figure 2c], an increase in cells in early apoptosis [Figure 2e], accumulation of OMP [Figure 3] and a decrease in PM [Figure 5a].
Younger stallions showed the lowest levels of DHE-positive spermatozoa [Figure 1d] and the lowest levels of MDA [Figure 4a]. A low concentration of superoxide may indicate low oxygen consumption by mitochondria in younger stallions, which is accompanied by a decrease in ATP synthesis [5], and a decrease in total and PM [Figure 5a and b].
Our data have shown that it is best to use the sperm of mature stallions for cryopreservation. To improve the outcome of cryopreservation in older stallions of high-value breeds, the use of antioxidants as a dietary supplement may be considered. Thus, Freitas et al. [50] showed that 60-day intake of nutraceuticals (antioxidants + fatty acids) improves the quality of stallions’ sperm, as evidenced by an increase in the integrity of sperm membranes and the kinetics of fresh, cooled, and frozen sperm. Numerous studies are also underway to test various antioxidants added to cryopreservation media, which can also be used to preserve valuable genetic material of elderly animals. Contreras et al. [51] showed that the addition of the antioxidant MnTBAP to the cryopreservation medium of stallions reduced the effects of oxidative stress and sperm damage after thawing, increasing plasma membrane integrity, mitochondrial potential, sperm motility, and sperm count. Poor sperm cryostability leads to financial and genetic waste in breeding programs. Antioxidant strategies could mitigate these losses, which could reduce losses in breeding programs. Nevertheless, since ROS plays a significant role in sperm capacitation and hyperactivation [9], the use of antioxidants to improve post-thaw sperm quality should be accompanied by parallel assessments of ROS levels and PM. In addition, uncontrolled antioxidant use may lead to reductive stress [52]. A promising approach would be to establish threshold ROS levels associated with optimal post-cryopreservation sperm quality.
The distinct phenotype observed in younger stallions – low oxidative damage coupled with low motility – points toward a deficiency in energy production rather than oxidative injury. This suggests that cryopreservation strategies for this age group should diverge from those designed for older animals. Instead of focusing on antioxidant defense, future research should test the efficacy of supplementing freezing extenders with energy substrates like pyruvate and lactate [53] to bolster ATP availability post-thaw. Furthermore, exploring mitochondria-targeted compounds such as L-carnitine [54] or MitoQ at low doses [55], may help enhance mitochondrial function without suppressing the essential ROS signaling that is already minimal in this group. Optimizing cooling protocols for the specific membrane composition of sperm from younger stallions could also yield significant improvements in cryosurvival.
4. CONCLUSION
Cryopreservation induces redox stress in stallion sperm, a process significantly influenced by donor age. Sperm from older stallions (16–24 years) display clear oxidative damage, leading to poor post-thaw motility. Younger stallions (3–5 years) show little oxidative injury yet also reduced motility, consistent with lower mitochondrial output. Mature stallions (6–15 years) demonstrate optimal post-thaw quality, supported by a balanced redox state, which makes them the best candidates for cryopreservation. These findings advocate for age-specific cryopreservation protocols: antioxidants for older and energy substrates for younger stallions, pending future validation to avoid reductive stress.
5. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines
6. FUNDING
This research was supported by the Ministry of Science and Higher Education of the Russian Federation (State Assignment Program No. FGZR-2025-0003).
7. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
8. ETHICAL APPROVALS
Ethical approvals details are given in the ‘Materials and Methods’ section.
9. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
10. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
11. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declare that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Naumenkova VA, Kalashnikov BB, Zaitsev AM, Atroshchenko MM, Kalashnikova TV. Dynamics of quality indicators of stallion sperm in related generations (material from the bioresource collection “cryobank of genetic resources“of the Federal State Budgetary Institution “All-Russian Research Institute of Horse Breeding“was used). Horse Breed Equestrian Sports. 2018;2:32-4.[CrossRef]
2. Atroshchenko MM, Bragina EE, Zaitsev AM, Kalashnikov VV, Naumenkova VA, Kudlaeva AM, et al. Conservation of genetic resources in horse breeding and major structural damages of sperm during semen cryopreservation in stallions. Nat Conserv Res. 2019;4(S2):78-82.[CrossRef]
3. Khan IM, Cao Z, Liu H, Khan A, Rahman SU, Khan MZ, et al. Impact of cryopreservation on spermatozoa freeze-thawed traits and relevance OMICS to assess sperm Cryo-tolerance in farm animals. Front Vet Sci. 2021;8:609180.[CrossRef]
4. Dowsett KF, Knott LM. The influence of age and breed on stallion semen. Theriogenology. 1996;46(3):397-412.[CrossRef]
5. Dar CR, Moraes LE, Scanlan TN, Baumber-Skaife J, Loomis PR, Cortopassi GA, et al. Sperm mitochondrial function is affected by stallion age and predicts post-thaw motility. J Equine Vet Sci. 2017;50:52-61.[CrossRef]
6. Aurich J, Kuhl J, Tichy A, Aurich C. Efficiency of semen cryopreservation in stallions. Animals (Basel). 2020;10(6):1033.[CrossRef]
7. Abah KO, Fontbonne A, Partyka A, Nizanski W. Effect of male age on semen quality in domestic animals:Potential for advanced functional and translational research. Vet Res Commun. 2023;47 (3):1125-37.[CrossRef]
8. Peña FJ, O'Flaherty C, Ortiz Rodríguez JM, Martín Cano FE, Gaitskell-Phillips GL, Gil MC, et al. Redox regulation and oxidative stress: The particular case of the stallion spermatozoa. Antioxidants (Basel). 2019;8(11):567. [CrossRef]
9. Serafini S, O'Flaherty C. Redox regulation to modulate phosphorylation events in human spermatozoa. Antioxid Redox Signal. 2022;37(7-9):437-450.[CrossRef]
10. Aitken RJ, Drevet JR, Moazamian A, Gharagozloo P. Male infertility and oxidative stress:A focus on the underlying mechanisms. Antioxidants (Basel). 2022;11:306.[CrossRef]
11. Atroshchenko MM, Medvedev DV. Biochemical markers of semen quality in stallions (review). Agric Biol. 2023;58(2):249-59.[CrossRef]
12. Naumenkov AI, Roman'kova NK. The method for stallion semen cryopreservation. In:Theoretical and Practical Aspects of Horse Breeding. Vol. 25. Divovo, Russia: Scientific reports of Russian Institute of Horse Breeding; Russian Institute of Horse Breeding;1971.128-132.
13. Koroliuk MA, Ivanova LI, Maiorova IG, Tokarev VE. Metod opredeleniia aktivnosti katalazy [A method of determining catalase activity]. Lab Delo. 1988;(1):16-9.
14. Kostiuk VA, Potapovich AI, Kovaleva ZV. Prostoi i chuvstvitel'nyimetod opredeleniia aktivnosti superoksid- dismutazy, osnovannyina reaktsii okisleniia kvertsetina [A simple and sensitive method of determination of superoxide dismutase activity based on the reaction of quercetin oxidation]. Vopr Med Khim. 1990;36(2):88-91.
15. Dubinina EE, Burmistrov SO, Khodov DA, Porotov IG. Okislitel'naia modifikatsiia belkov syvorotki krovi cheloveka, metod ee opredeleniia [Oxidative modification of human serum proteins. A method of determining it]. Vopr Med Khim. 1995;41(1):24-6.
16. Shitikova AM, Atroshchenko MM, Zvyagina VI. Thiol cathepsins and oxidative modification of stallion's seminal plasma proteins with normal and low percentage of live spermatozoa post cryopreservation. Indian J Anim Res. 2024;58(10):1658-64.[CrossRef]
17. Fomina MA, Abalenikhina YuV, Fomina NV, Terent'ev AA. Method of Complex Assessment of the Content of Oxidative Protein Modification Products in Tissues and Biological Fluids. Russian Patent no:2524667;2014.
18. Castro R, Morales P, Parraguez VH. Post-thawing sperm quality in Chilean Purebred Stallions:Effect of age and seasonality. J Equine Vet Sci. 2020;92:103170.[CrossRef]
19. Greiser T, Sieme H, Martinsson G, Distl O. Breed and stallion effects on frozen-thawed semen in warmblood, light and quarter horses. Theriogenology. 2020;142:8-14.[CrossRef]
20. Arifin WN, Zahiruddin WM. Sample Size Calculation in Animal Studies Using Resource Equation Approach. Malays J Med Sci. 2017;24(5):101-5.[CrossRef]
21. Ozkavukcu S, Erdemli E. Cryopreservation:Basic knowledge and biophysical effects. J Ankara Med Sch. 2002;24(4):187-96.
22. Pasciu V, Nieddu M, Sotgiu FD, Baralla E, Berlinguer F. An overview on assay methods to quantify ROS and enzymatic antioxidants in erythrocytes and spermatozoa of small domestic ruminants. Animals. 2023;13(14):2300. [CrossRef]
23. Vašícek J, Baláži A, SvoradováA, Vozaf J, DujíckováL, Makarevich AV, et al. Comprehensive flow-cytometric quality assessment of ram sperm intended for gene banking using standard and novel fertility biomarkers. Int J Mol Sci. 2022;23(11):5920.[CrossRef]
24. Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, et al. Measuring reactive oxygen and nitrogen species with fluorescent probes:Challenges and limitations. Free Radical Biol Med. 2012;52(1):1-6. [CrossRef]
25. Burnaugh L, Sabeur K, Ball BA. Generation of superoxide anion by equine spermatozoa as detected by dihydroethidium. Theriogenology. 2007;67(3):580-589.[CrossRef]
26. Cocuzza M, Athayde KS, Agarwal A, Sharma R, Pagani R, Lucon AM, et al. Age-related increase of reactive oxygen species in neat semen in healthy fertile men. Urology. 2008;71(3):490-4.[CrossRef]
27. Ortega-Ferrusola C, Anel-Lopez L, Martin-Munoz P, Ortiz-Rodriguez JM, Gil MC, Alvarez M, et al. Computational flow cytometry reveals that cryopreservation induces spermptosis but subpopulations of spermatozoa may experience capacitation-like changes. Reproduction. 2017;153(3):293-304.[CrossRef]
28. Koppers AJ, Mitchell LA, Wang P, Lin M, Aitken RJ. Phosphoinositide 3-kinase signalling pathway involvement in a truncated apoptotic cascade associated with motility loss and oxidative DNA damage in human spermatozoa. Biochem J. 2011;436(3):687-98. [CrossRef]
29. O'Flaherty C, Matsushita-Fournier D. Reactive oxygen species and protein modifications in spermatozoa. Biol Reprod. 2017;97(4):577-85.[CrossRef]
30. Akagawa M. Protein carbonylation:Molecular mechanisms, biological implications, and analytical approaches. Free Radical Res. 2021;55(4):307-320.[CrossRef]
31. Rudzinska M, Parodi A, Balakireva AV, Chepikova OE, Venanzi FM, Zamyatnin AA Jr. Cellular aging characteristics and their association with age-related disorders. Antioxidants (Basel). 2020;9(2):94.[CrossRef]
32. Faletrova SV, Uryas'yev OM, Bel'skikh ES. Investigation of effect of adipose tissue on carbonyl stress markers in patients with chronic obstructive pulmonary disease in non-infectious exacerbation. Sci Young (Eruditio Juvenium). 2024;12(1):45-54.[CrossRef]
33. Atroshchenko MM, Engalycheva MG, Shitikova AM. Assessment of oxidative modification of proteins of the spermoplasm of stallions (Equus ferus caballus L.) of different ages. Agric Biol. 2023;58(4):660-8.[CrossRef]
34. Shitikova AM, Atroshchenko MM, Zvyagina VI, Shirokova OV, Frolova NA, Strokova M. Oxidative status of peripheral blood mononuclear cells of stallions of different ages. Indian J Anim Res. 2024;59:1310-6.[CrossRef]
35. Sharapov MG, Gudkov SV, Lankin VZ. Hydroperoxide-reducing enzyme systems in the regulation of free radical processes. Biochemistry. 2021;86(10):1479-501.[CrossRef]
36. Kumar M, Ranjan R, Bhardwaj A. Effects of liquid storage of buck semen at refrigeration temperatures on sperm viability and fertility to develop ready to use goat semen diluent. J Appl Biol Biotechnol. 2024;12(5):114-8.[CrossRef]
37. Chernykh IV, Shchul'kin AV, Popova NM, Gatsanoga MV, Yakusheva EN. Regulation of ABCB1 protein function in the cerebral cortex with the underlying global cerebral ischemia. I. P. Pavlov Russ Med Biol Herald. 2023;31(4):613-622.[CrossRef]
38. Catalán J, Yánez-Ortiz I, Torres-Garrido M, Ribas-Maynou J, Llavanera M, Barranco I, et al. Impact of seminal plasma antioxidants on DNA fragmentation and lipid peroxidation of frozen-thawed horse sperm. Antioxidants (Basel). 2024;13(3):322.[CrossRef]
39. Oddi S, Carluccio A, Ciaramellano F, Mascini M, Bucci R, Maccarrone M, et al. Cryotolerance of equine spermatozoa correlates with specific fatty acid pattern:A pilot study. Theriogenology. 2021;172:88-94.[CrossRef]
40. Leonard AE, Kelder B, Bobik EG, Chuang LT, Lewis CJ, Kopchick JJ, et al. Identification and expression of mammalian long-chain PUFA elongation enzymes. Lipids. 2002;37(8):733-40.[CrossRef]
41. Gaitskell-Phillips G, Martín-Cano FE, Ortiz-Rodríguez JM, Silva-Rodríguez A, Gil MC, Ortega-Ferrusola C, et al. In stallion spermatozoa, superoxide dismutase (cu-zn) (sod1) and the aldo-keto-reductase family 1 member b (AKR1B1) are the proteins most significantly reduced by cryopreservation. J Proteome Res. 2021;20(5):2435-2446.[CrossRef]
42. Selvaratnam JS, Robaire B. Effects of aging and oxidative stress on spermatozoa of superoxide-dismutase 1- and catalase-null mice. Biol Reprod. 2016;95(3):60.[CrossRef]
43. Selvaratnam J, Robaire B. Overexpression of catalase in mice reduces age-related oxidative stress and maintains sperm production. Exp Gerontol. 2016;84:12-20.[CrossRef]
44. Oehninger S, Franken DR, Sayed E, Barroso G, Kolm P. Sperm function assays and their predictive value for fertilization outcome in IVF therapy:A meta-analysis. Hum Reprod Update. 2000;6(2):160-8.[CrossRef]
45. Srivastava N, Pande M. Protocols in Semen Biology (Comparing Assays). Singapore:Springer;2017. [CrossRef]
46. Medica AJ, Swegen A, Seifi-Jamadi A, McIntosh K, Gibb Z. Catalase in unexpected places:Revisiting H2O2 detoxification pathways in stallion spermatozoa. Antioxidants (Basel). 2025;14(6):718.[CrossRef]
47. Evdokimov VV, Barinova KV, Turovetskii VB, Muronetz VI, Schmalhausen EV. Low concentrations of hydrogen peroxide activate the antioxidant defense system in human sperm cells. Biochemistry. 2015;80:1178-85. [CrossRef]
48. Aitken R, Lambourne S, Gibb Z. The John Hughes memorial lecture:Aspects of sperm physiology-oxidative stress and the functionality of stallion spermatozoa. J Equine Vet Sci. 2014;34(1):17-27.[CrossRef]
49. Gibb Z, Griffin RA, Aitken RJ, De Iuliis GN. Functions and effects of reactive oxygen species in male fertility. Anim Reprod Sci. 2020;220:106456.[CrossRef]
50. Freitas M, Bouéres CS, Pignataro TA, De Oliveira FJ, De Oliveira Viu MA, De Oliveira RA. Quality of fresh, cooled, and frozen semen from stallions supplemented with antioxidants and fatty acids. J Equine Vet Sci. 2016;46:1-6.[CrossRef]
51. Contreras MJ, Treulen F, Arias ME, Silva M, Fuentes F, Cabrera P, et al. Cryopreservation of stallion semen:Effect of adding antioxidants to the freezing medium on sperm physiology. Reprod Domest Anim. 2020;55(2):229-39.[CrossRef]
52. Moustakli E, Zikopoulos A, Skentou C, Katopodis P, Domali E, Potiris A, et al. Impact of Reductive stress on human infertility:Underlying mechanisms and perspectives. Int J Mol Sci. 2024;25(21):11802.[CrossRef]
53. Darr CR, Varner DD, Teague S, Cortopassi GA, Datta S, Meyers SA. Lactate and pyruvate are major sources of energy for stallion sperm with dose effects on mitochondrial function, motility, and ROS production. Biol Reprod. 2016;95(2):34.[CrossRef]
54. Palacios P, Peláez G, Soria M, Méndez S, Galarza-Álvarez L, Dorado J, et al. L-carnitine enhances the kinematics and protects the sperm membranes of chilled and frozen-thawed Peruvian Paso horse spermatozoa. Cryobiology. 2024;115:104884.[CrossRef]
55. Elkhawagah AR, Donato GG, Poletto M, Martino NA, Vincenti L, Conti L, et al. Effect of mitoquinone on sperm quality of cryopreserved stallion semen. J Equine Vet Sci. 2024;141:105168.[CrossRef]