1. INTRODUCTION
The Sahara is the largest hot desert in the world, which is located in northern Africa [1]. It makes up more than 30% of the continent’s surface and covers more than 8.6 million square kilometers [2]. It extends roughly 5,000 km from the Red Sea in the east to the Atlantic Ocean in the west, and between 1,300 and 1,900 km from north to south, covering significant portions of Algeria, Egypt, Libya, Mali, Morocco, Mauritania, Niger, Sudan, Chad, and Tunisia [3,4]. The Sahara Desert is a parched area where temperatures may ascend to 58°C, and yearly rainfall is exceedingly minimal, averaging about 25.4 mm, resulting in severely restricted vegetation cover [5,6].
The flora adapted to the Sahara Desert includes grasses, shrubs, and even trees, particularly in oases and along wadis. The majority of these plants are halophytes [7-9]. Many species that typically grow in higher latitudes and altitudes can also be found in the Saharan region, such as olive, cypress, pistachio, Acacia [10], Artemisia [9], doum palm, date palm [11], and thyme [12].
With a large number of endemic species and numerous plant groups, such as Amaranthaceae, Brassicaceae, Poaceae, Fabaceae, Boraginaceae, Asteraceae, and Lamiaceae, Morocco’s Saharan region is known for its diverse flora [13]. These families include several species that are used in traditional medicine, nutrition, and as food additives due to their known medicinal qualities [14,15]. These plants are frequently used by local populations to treat a variety of illnesses, such as diabetes [18], skin conditions, oral infections [19], digestive disorders [16,17], and even venomous bites and stings [20]. The existence of bioactive substances with proven antibacterial, anti-inflammatory, and antioxidant properties is usually associated with these therapeutic uses [17].
Flavonoids and other phenolic compounds are essential to the pharmacological and therapeutic properties of plants, particularly due to their strong antioxidant activity. These compounds exhibit various biological activities, such as cardioprotective, neuroprotective, anti-inflammatory, and anti-cancer effects. Numerous studies support their potential for drug development. For instance, species high in phenols and flavonoids may be promising substitutes for the medicinal plants that have traditionally been used to treat breast cancer in Côte d’Ivoire. By affecting biological pathways connected to inflammation and oxidative stress, these compounds could assist in the treatment of several illnesses, including cancer, according to similar findings from another study. Overall, the data highlight their pharmacological worth and their applicability to pharmaceutical research and novel treatment approaches [21,22]. Further investigation into the specific mechanisms by which phenols and flavonoids exert their effects will be vital for maximizing their use in clinical settings. Understanding these pathways could lead to the development of more targeted therapies that enhance patient outcomes while minimizing side effects [21,22].
This study seeks to investigate the flora of southern Morocco, emphasizing the significance of aromatic and medicinal plants (AMPs) in Saharan traditional practices. An ethnobotanical inquiry was undertaken to catalog the typical flora of each region and their traditional use. In addition, the chemical composition and antioxidant potential of selected species were assessed.
2. MATERIALS AND METHODS
2.1. Study Sites
The investigated areas are predominantly plains characterized by a hot, arid climate, and minimal annual rainfall. Floristic diversity and dominant species vary from one locality to another. A total of 17 sites were surveyed to identify the existing and dominant plant species [Figure 1].
 | Figure 1: Map of the 17 study sites. [Click here to view] |
The geographical coordinates of each study site are presented in Table 1.
Table 1: Geographical coordinates of surveyed sites
| Site | GPS coordinates |
|---|---|
| AR-01 | 23°59’2’’ N 15°87’7’’ O |
| AR-02 | 23°39’15’’ N 15°99’09’’ O (tropic of cancer) |
| AR-03 | 22°55’ N 16°11’14’’ O |
| AR-04 | 22°28’57’’ N 16°26’14’’ O |
| AR-05 | 22°55’02’’ N 16°52’17’’ O |
| AR-06 | 21°22’27’’ N 16°57’36’’ O |
| AR-07 | 23°14’ N 15°55’ O |
| AR-08 | 23°38’19’’ N 15°41’11’’ O |
| AR-09 | 23°15’20’’ N 15°10’13’’ O |
| AR-10 | 22°33’18’’ N 14°19’1’’ O |
| AR-11 | 24°1’12’’ N 15°35’57’’ O |
| AR-12 | 24°13’45’’ N 15°25’24’’ O |
| AR-13 | 24°55’3’’ N 14°47’54’’ O |
| AR-14 | 26°12’8’’ N 14°21’53’’ O |
| AR-15 | 26°24’58’’ N 14°2’5’’ O |
| AR-16 | 26°32’23’’ N 13°50’16’’ O |
| AR-17 | 27°3’11’’ N 13°24’48’’ O |
2.2. Plant Material
In March 2022, 29 medicinal and aromatic plants from various botanical families were gathered from 17 locations in the southernmost region of Morocco, Dakhla-Oued Ed-Dahab. Sampling was carried out along the road linking Dakhla to Guerguerat, passing through Aousserd and Sebkhet Imlili. At the same time, the samples were labeled and stored in paper bags. The collected samples were sent for botanical identification to the Scientific Institute of Rabat, Department of Botany and Plant Ecology, Mohammed V University. Botanist Mr. Mohammed Fenane, using his manual entitled “Practical Flora of Morocco,” carried out the identification. Plant names were further verified using the World Flora Online database (www.worldfloraonline.org). Voucher specimens of each identified plant were deposited in the National Herbarium of the Scientific Institute of Rabat. Then, plants were air-dried in the dark at room temperature, ground into a fine powder using an electric mill, and stored in dark glass bottles for phytochemical and antioxidant analyses.
2.3. Ethnobotanical Survey
A total of 50 informants, 32 women and 18 men, aged between 25 and 80, were selected for the ethnobotanical survey based on their traditional knowledge of medicinal plants in the region, including herbalists, elderly residents, and shepherds encountered i during field investigation. Data were collected using a questionnaire during the interview. The information on the questionnaire focused on the local plant names, the parts used, the preparation methods, the therapeutic indications, and traditional recipes. The ethnobotanical survey was conducted in accordance with the ethical principles of the International Society of Ethnobiology and following COPE/ICMJE guidelines. All informants provided their verbal consent before participation, and anonymity and confidentiality were fully guaranteed.
2.4. Phytochemical Extraction and Analysis
2.4.1. Extraction
To prepare the leaf extracts, 2.5 g of dried plant material was dissolved in 50 mL of 80% methanol (methanol: water, 8:2 v/v). The samples were extracted twice to maximize compound recovery from the samples. The mixture was stirred using a magnet for 15 min, followed by ultrasonic treatment for another 15 min. It was filtered and spun in a centrifuge at 3500 rpm for 15 min after the solution had settled. The supernatant was then stored at 4°C for further analysis.
2.4.2. Thin layer chromatography (TLC)
TLC was employed to separate the constituents of the methanolic plant extracts and to gain semi-quantitative insight into their chemical composition. A volume of 10 μL from each extract was carefully spotted onto silica gel chromatoplates. A solvent mixture, consisting of ethyl acetate, formic acid, acetic acid, and water (100:11:11:27, v/v), was used for chromatographic separation. Following development, the plates were exposed to 1% 2-aminoethyl-diphenylborinate in methanol (Neu’s reagent) and evaluated at 365 nm under a UV lamp [23].
2.4.3. Total phenol determination
The Folin–Ciocalteu method was applied to determine total phenolic content [24]. For each analysis, 1 mL of plant extract was combined with 1 mL of 80% methanol, 5 mL of distilled water, and 0.5 mL of Folin–Ciocalteu reagent diluted tenfold. After a 5-min incubation, 1 mL of 5% sodium carbonate was added, and the mixture was vortexed. The samples were then kept in the dark for 30 min to allow color development. Absorbance was read at 725 nm with an IC 6400 spectrophotometer. Phenolic content was calculated from a gallic acid standard curve and expressed as μg gallic acid equivalents (GAE) per mg of dry matter. There were three replicates of each measurement.
2.4.4. Total flavonoid determination
Neu’s reagent [23], a boron-based substance that forms fluorescent complexes with flavonoids, was used to measure the total flavonoid content in accordance with the procedure outlined by Andary [24]. To start the reaction, 100 μL of Neu’s reagent was combined with 2 mL of each extract (1% concentration). An IC 6400 UV-visible spectrophotometer was used to measure the optical density at 404 nm. The calibration curve was created concurrently using a quercetin solution (0.05 mg/mL).
The following formula was then used to quantify the flavonoid content, as described by [25]:
 |
In this equation, Aext represents the absorbance of the extract, Aq corresponds to the absorbance of the quercetin standard (0.05 mg/mL), and Cext denotes the extract concentration expressed in mg/mL. Each sample was analyzed in triplicate, and results were reported as milligrams of quercetin equivalents per gram of dry matter (mg QE/g DM).
2.4.5. Antioxidant activity (2,2-diphenyl-1-picrylhydrazyl [DPPH] free radical scavenging activity) determination
Free radical scavenging activity was assessed using the DPPH assay. In the presence of free radical scavengers, the purple-colored DPPH compound is reduced to the yellow-colored 2,2-diphenyl-1-picrylhydrazine [26,27].
The effect of plant extracts on DPPH radicals was evaluated following the method described by Masuda et al. [28]. Briefly, 950 μL of a methanolic solution containing 0.1 mM DPPH was mixed with 50 μL of the sample. Following a 30-min incubation period, the absorbance of each sample was recorded at 517 nm. A reduction in absorbance reflected the ability of the extract to scavenge DPPH free radicals. The inhibition percentage was then determined using the following equation [29]:
 |
In this equation, P is the percentage of DPPH inhibition, A1 is the absorbance of the control (DPPH solution without extract), and A2 is the absorbance measured when the extract is present. We tested each sample 3 times at different concentrations to find the IC50 value, which is the concentration needed to stop 50% of DPPH radicals.
2.5. Statistical Analysis
The data from the statistical analysis were shown as the mean ± standard error of three copies. OriginPro software version 2025 (OriginLab Corporation, Northampton, MA, USA) was used to do a one-way analysis of variance (ANOVA) to see how important differences in total phenolic content, flavonoid content, and antioxidant activity (IC50) were between the species studied. After revealing significant differences (P < 0.05), Tukey’s post-hoc test was applied to categorize the species according to analogous statistical behavior. In addition, Pearson correlation analysis was performed to examine the relationships between phenolic content, flavonoid content, and antioxidant activity.
3. RESULTS
3.1. Collecting and Monographing of Spontaneous AMPs
The study region is characterized by a Saharan climate featuring low and irregular rainfall, exacerbated by high temperatures and continuous winds. These environmental circumstances support the growth of plant species adapted to aridity in the desert, such as Acacia raddiana, Zygophyllum gaetulum, Launaea arborescens, Anastatica hierochuntica, and Rhus tripartita, among others. The floristic diversity observed at the studied sites, including dominant and harvested species, is represented in Table 2. Plant diversity was documented within each surveyed site and across the region. Sites 6, 13, 16, and 17 exhibit notably high plant diversity, whereas sites 1, 4, 7, 9, 10, 11, and 15 show moderate levels of diversity. Several other sites exhibited low plant diversity, typically marked by the dominance of a single species [Table 2].
Table 2: Characterization of the surveyed sites
| Sites | Floristic diversity | Dominant species | Harvested species |
|---|---|---|---|
| AR-01 | Average | Zygophylum gaetulum Emb. & Maire, Frankenia corymbosa Desf. | Atriplex halimus L., Frankenia corymbosa Desf. , Launaea arborescens (Batt.) Murb, Zygophylum gaetulum Emb. & Maire |
| AR-02 | Low | Zygophylum gaetulum Emb. & Maire, Frankenia corymbosa Desf. | Salsola tetrandra Forssk., Salsola tetragona Delile |
| AR-03 | Low | Tamarix amplexicaulis Ehrenb. | Tamarix amplexicaulis Ehrenb. |
| AR-04 | Average | Frankenia corymbosa Desf. | Limonium tuberculatum (Boiss.) Kuntze, Frankenia corymbosa Desf., salsola sp |
| AR-05 | Low | Mesembryanthemum theurkauffii (Maire) Maire | Mesembryanthemum theurkauffii (Maire) Maire |
| AR-06 | Important | Zygophylum gaetulum Emb. & Maire, Launaea arborescens (Batt.) Murb. | Nitraria retusa (Forssk.) Asch. , Heliotropium erosum Lehm. , Farsetia occidentalis B.L.Burtt, Lotus chazaliei H.Boissieu, Zygophyllum gaetulum Emb. & Maire, Launaea arborescens (Batt.) Murb., Lotus cornicatus L., Haloxylon scoparium Pomel |
| AR-07 | Average | Nitraria retusa (Forssk.) Asch. | Arthrocnemum macrostachyum (Moric.) K.Koch, Nitraria retusa (Forssk.) Asch. |
| AR-08 | Low | Acacia ehrenbergiana Hayne | Acacia ehrenbergiana |
| AR-09 | Average | Acacia ehrenbergiana Hayne | Acacia ehrenbergiana, Acacia raddiana SaviHeliotropium erosum Lehm., Pergularia tomentosa L. |
| AR-10 | Average | Ziziphus lotus. subsp saharae (Batt. & Trab.) Maire | Ziziphus lotus. subsp saharae (Batt. & Trab.) Maire, Senna italica Mill. |
| AR-11 | Average | Launaea arborescens (Batt.) Murb., Zygophyllum gaetulum Emb. & Maire | Launaea arborescens (Batt.) Murb., Zygophyllum gaetulum Emb. & Maire, Atriplex halimus L. |
| AR-12 | Low | Salsola sp. | Salsola sp. |
| AR-13 | Important | Anastatica hierochuntica L., Euphorbia officinarum. subsp echinus L. | Lycium intricatum Boiss. , Deverra denudata (Viv.) Pfisterer & Podlech, Anastatica hierochuntica L., Euphorbia officinarum. subsp echinus L. |
| AR-14 | Low | Euphorbia officinarum. Subsp echinus L. | latex d’euphorbia officinarum. Subsp echinus L. |
| AR-15 | Average | Rhus tripartita Moffet | Rhus tripartita Moffet, Deverra denudata (Viv.) Pfisterer & Podlech |
| AR-16 | Important | Frankenia corymbosa Desf. , Sedum sediforme (Jacq.) Pau, Zygophyllum gaetulum Emb. & Maire | Frankenia corymbosa Desf., Sedum sediforme (Jacq.) Pau, Spergularia diandra (Guss.) Boiss., Zygophyllum gaetulum Emb. & Maire |
| AR-17 | Important | Launaea arborescens (Batt.) Murb. | Heliotropium erosum Lehm., Launaea arborescens (Batt.) Murb., Frankenia corymbosa Desf., Salsola sp |
The species Z. gaetulum, F. corymbosa, and L. arborescens were observed in four different sites, whereas species such as R. tripartita and T. amplexicaulis were exclusively found at a single site each. Sites 1 and 2 showed similarities in terms of species dominance. A. hierochuntica, renowned for its traditional uses, was prominently observed in site 13, where it was prevalent alongside Euphorbia officinarum subsp. echinus. Mesembryanthemum theurkauffii, a succulent species, was exclusively observed only at site 5. Site 12, located in the Aousserd region, exhibited sparse and relatively rare vegetation, reflecting the challenging climatic conditions for plant survival. Only one species from the genus Salsola was identified at this site.
The collected species were categorized into 19 families [Figure 2], most of which are native and some endemic. The Amaranthaceae family ranks first with 5 species, followed by Fabaceae with four species, and then Apocynaceae, Brassicaceae, and Solanaceae with two species each. Each of the remaining families is represented by one species.
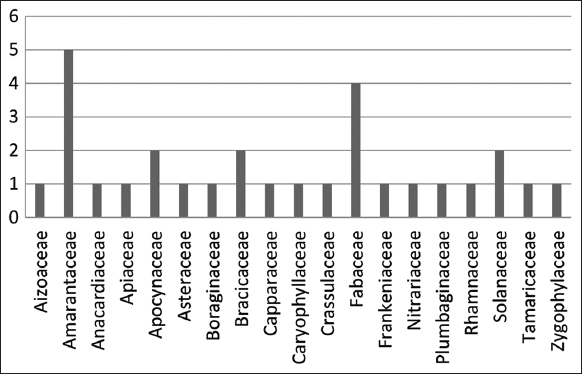 | Figure 2: The number of species collected per family. [Click here to view] |
The ethnobotanical investigation revealed that the local population makes extensive use of harvested plant species in traditional medicine to address a wide range of ailments. Digestive disorders emerged as the most commonly treated condition, representing 25.71% of reported uses, followed by skin infections (20%) and diabetes (11.43%) [Figure 3]. In addition, several species were cited for managing obesity, cancer, oral infections, infertility, hair-related issues, and poisoning. Conversely, ailments such as rheumatism, joint pain, and liver conditions were less frequently mentioned by respondents [Figure 3], suggesting either limited traditional knowledge or fewer perceived effects of the plants on these conditions.
 | Figure 3: Frequency of use of the plants species to treat diverse diseases. [Click here to view] |
According to the results of our survey, leaves are the most frequently used part of the various species in the preparation of traditional remedies, accounting for 53.85% of uses. This is followed by stems, roots, whole plants, and other parts such as seeds, bark, latex, and gum [Figure 4].
 | Figure 4: Frequency of use of various parts of the plant species by the local population. [Click here to view] |
To prepare their traditional remedies, the local population uses various methods, including decoction, infusion, and powder form. According to the ethnobotanical survey results, the majority of remedies are prepared as decoctions, accounting for 63.16% of preparations, followed by dried powder at 26.32%, and infusions at 10.53% [Figure 5].
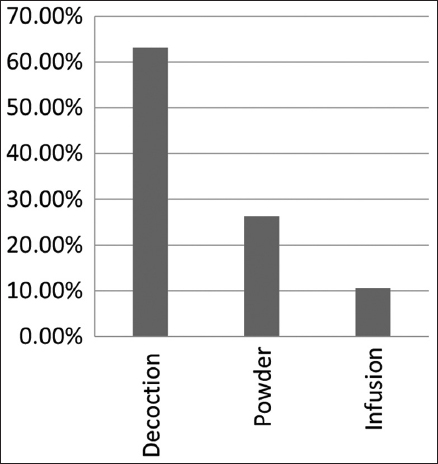 | Figure 5: Frequency of use of different methods of preparing plants by the local population. [Click here to view] |
The ethnobotanical survey and the collection of spontaneous plants carried out in this study enabled the creation of a monograph compiling data on the 29 collected species. This monograph includes information such as families, scientific names, vernacular names, local names, parts used, preparation methods, traditional uses, and bibliographic references [Table 3].
Table 3: Monograph of the aromatic and medicinal plants collected.
| Family | Scientific name | French name | Local name | Part used | Local uses | Method of preparation | Traditional use |
|---|---|---|---|---|---|---|---|
| Aizoaceae | Mesembryanthemum theurkauffii (Maire) Maire | Afzou | -Leaf | - Reduction of body fat - Treatment of infertility - Treatment of diabetes | Decoction | Seed: - Preparation of flour to make pancakes [30] | |
| Amaranthaceae (=Chenopodiaceae) | Atriplex halimus L. | Arroche halime, pourpier de mer | Legdef | -Leaf - Seed | - Cancer treatment - Treatment of diabetes - Treatment of digestive problems - Emetic -Oral care | Decoction | Whole plant: - Treatment of insomnia and gastric diseases[30] - Treatment of diarrhea and inflammation[31] Seed: - Emetic[30] - Treatment of diabetes and cancer [32] |
| Salsola tetragona Delile | Salsole | Aqssal, laarad | -Aerial part or leaves | - Treatment of digestive problems - Treatment of skin infections | Decoction | Whole plant: - Treatment of stomach ailments - Treatment of pimples and ringworm - Washing hair, tanning skins - Source of fuel, degreasing of linen and wool fleeces. - Treatment of hypertension -Treatment of inflammation - Treatment of jaundice - Treatment of insomnia - Diuretic [30,33] | |
| Salsola tetrandra Forssk. | Salsole | Aqssal | -Aerial part orleaves | - Treatment of digestive problems - Treatment of skin infections | Decoction | Whole plant: - Treatment of gastric disorders - Treatment of pimples and ringworm - Hair washing, hide tanning - Fuel source, degreasing linen, and wool fleeces. - Treatment of hypertension - Treatment of inflammation - Treatment of jaundice [30,33] | |
| Haloxylon scoparium Pomel | Saligne à balai | Remt | -Leaf | - Cancer treatment | Powder (Oral consumption of leaf powder mixed with euphorbia honey) | Whole plant: - Treatment of herd mange (decoction with tobacco juice) Anti-poison (decoction) -Treatment of wounds (powder) - Treatment of diabetes (powder) Fruit pericarp and stem: - Cataplasms in snakebite treatment (powder mixed with fat) Root: - Treatment of digestive disorders (powder) [30,34,35] | |
| Arthrocnemum macrostachyum (Moric.) K.Koch | Salicorne glauque | ND | ND | ND | Whole plant: - Treatment of diabetes - Treatment of snake bites and scorpion stings (powder) -Hypoglycemic [36] | ||
| Anacardiaceae | Rhus tripartita Moffet | Jdari | -Bark - Leaf | - Treatment of digestive problems | Decoction or powder (oral consumption of powder with water) | Fruit, leaf, and bark: - For colic, gastric disorders and ulcers (decoction) Wood: - Toothbrush Bark and leaf:1 - Tinting (powder mixed with henna paste) [30] | |
| Apiaceae | Deverra denudata (Viv.) Pfisterer and Podlech | Gziziha | ND | ND | ND | Fruit: - Treatment of fever (decoction) Whole plant: - Digestive, carminative and diuretic - Relieves thirst [30]. | |
| Apocynaceae | Pergularia tomentosa L. | Pergulaire | Oum-Eljloud | ND | ND | ND | ND |
| Calotropis procera (Aiton) W.T. Aiton | Pomme de sodome | Tourza | ND | ND | ND | Leaf: - Vermifuge (low-dose powder) Latex: - Purgative (ingested in semolina porridge) - For warts and calluses, ringworm and alopecia - To remove thorns from the skin [30,37] | |
| Asteraceae | Launaea arborescens (Batt.) Murb. | Launée arborescente | Oum lbina | -Upper -Latex - Whole plant - Root | - Treatment of deworming - Treatment of skin infections such as pimples - Extraction of thorns - Feeding animals | Infusion | Latex: - Maturates or desiccates boils and pimples (direct application) - Facilitates extraction of thorns (direct application)[30] Whole plant: - Vermifuge for children (infusion) [30,38] -Treatment of gastric problems[39] - Treatment of rashes[32] - Treatment of gastroenteritis -Treatment of diabetes - Treatment of childhood illnesses (maceration or decoction)[40] - Treatment of diarrhea - Treatment of spasms [38] |
| Boraginaceae | Heliotropium erosum Lehm. | Héliotrope | Lhbaliya | -Leaf | - Treatment of abscesses, edema and burns | Decoction or powder | Whole plant: - Against poisoning [32] |
| Brassicaceae | Anastatica hierochuntica L. | Anastatique, Rose de Jéricho | Lkemcha | - Whole plant | - Treatment of rheumatism - Treatment of microbial infections of the uterus - Treatment of joint pain | Decoction and powder (In water and as an ointment with lhmira and goat butter) | Whole plant: - Used by midwives, eases childbirth, and soothes pain (oral maceration). - Treatment of cold (infusion or powder with honey and olive oil). Seed and whole plant:2 - Treatment of conjunctivitis - Improves hair retention (added to ghassul) [41-44]3 |
| Farsetia occidentalis B.L.Burtt | Farsétie | ND | ND | ND | ND | ||
| Capparaceae | Maerua crassifolia Forssk. | Atyle | -Stem - Leaf | - Oral care - Treatment of digestive problems - Treatment of toothache - Hair care | Decoction | Leaf: - Rapid wound healing (powder mixed or not with henna and dromedary fat as an ointment) - Reduction of fractures and sore spots (powder as a poultice) - Treatment of gastrointestinal and liver disorders (infusion administered orally) - Protection against microbes [30,45] Wood: - Making a traditional toothbrush (miswak)[30] Leaves and bark:4 - Treatment of fever -Treatment of headaches - Treatment of toothache (Decoction, crushed together and compressed on temples and jaws) -Treatment of scalp disorders [30] | |
| Caryophyllaceae | Spergularia diandra (Guss.) Boiss. | Spergulaire | ND | ND | ND | ND | |
| Crassulaceae | Sedum sediforme (Jacq.) Pau | Orpin de Nice | ND | ND | ND | ND | |
| Fabaceae | Lotus chazaliei H.Boissieu | Lotus sacré | Tamagchicht | ND | ND | ND | ND |
| Lotus corniculatus L. | Pied de poule | ND | ND | ND | ND | ||
| Senna italicaa Mill. | Aflajit | -Leaf | - Treatment of digestive problems | Infusion (Leaf powder added to leaves, marjoram and acacia gum infused in water) | Leaf and seed: - As a laxative - As purgative - As anti-icterus (Infusion) - As a dry eyewash (fine seed powder added to khol or used on its own) [30,46,47]5 | ||
| Acacia tortilis ssp.raddiana Savi | raddiana | Telh | -Fruit - Leaf - Gum | -Anti obesity - Hypoglycemic - Treatment of digestive disorders | Decoction (fruit powder mixed with rose leaves and mystic cumin) | Gum: - Eye drops to treat eye diseases (dissolved in water) - Treatment of pulmonary diseases (orally) - Treatment of oral and pharyngeal inflammations (orally). Root: - To treat stomach disorders Branch bark: - Disinfection and wound healing (powder) Seed: - Treatment of diarrhea (whole or ground) [30,48-50] | |
| Frankeniaceae | Frankenia corymbosa Desf. | Frankenie en corymbe | ND | ND | ND | ND | |
| Nitrariaceae | Nitraria retusa (Forssk.) Asch. | Nitraire à feuilles rétuses | Lgerzim | -Leaf | - Treatment of digestive problems - Treatment of poisoning - Drying pimples | Decoction | Leaf: - Tea preparation[51] - Against colds - Treatment of stomach ailments and poisoning (decoction administered orally) - To reduce swelling and dry pimples (fresh leaves as poultices) [30] |
| Plumbaginaceae | Limonium tuberculatum (Boiss.) Kuntze | Statice | Lgarssa | ND | ND | ND | Whole plant: - Diuretic (decoction) [30]. |
| Rhamnaceae | Ziziphus lotus subspsaharae (Batt. and Trab.) Maire | Jujubier du Sahara | Sder | -Leaf -Root | - Treatment of skin infections - Stimulation of hair growth - Treatment of diabetes - Treatment of boils and abscesses - Treatment of smallpox and measles | Decoction of roots in water | Fruit: - Antidiabetic, sedative, bronchitis, antidiarrheal, and febrifuge - Treatment of cystitis (combined with rush fruit, lavender, corn, couch grass, and prickly pear flowers) Leaf and fruit: - Treatment of boils and abscesses (powdered with sour milk or water, then applied as plasters) [30,52-54] |
| Solanaceae | Hyoscyamus muticus L. | Jusquiame d’Egypte | falezlez | ND | ND | ND | Whole plant: - Sedative and anaesthetic properties (internally and externally) [30,55] - Anticancer[56] Seed: - Aphrodisiac - Treatment of toothache (gargle the decoction) - Sedative and antispasmodic for bladder pain (in low doses) -Hypnotic for insomnia [30] |
| Lycium intricatum Boiss. | Lyciet | Lgherdeg | ND | ND | ND | Whole plant: - Eye drops for albugo and various other ophthalmic conditions - Protection against smallpox (poultice) Wood: - Antitussive and antitubercular (decoction) Anti-venomous and anti-rabies (internally) Anti-bite (rubbed externally) - Treatment of sterility in women (powder, vaginally) Fruit: -Gum strengthening (chewed) - Treatment of tonsillitis and mouth ulcers (gargle decoction) - Treatment of diabetes [30]. | |
| Tamaricaceae | Tamarix amplexicaulis Ehrenb. | Tamaris très ramifié | Tarfa | -Leaf | ND | ND | Bark: - Treatment of diarrhea (internal powder) - Gingival tonic (gargle the decoction) Hemostatic and anti-hemorrhoidal (sprinkles and poultices) [30] |
| Zygophyllaceae | Zygophylum gaetulum Emb. and Maire | Zygophylle du Maroc | laagaya | -Leaf - Flower | - Treatment of skin infections (Pimples and burns) - Treatment of digestive problems - Treatment of liver problems | Powder and decoction (Cutaneous application of powder) | Whole plant: - Treatment of diabetes[30] -Diuretic[57] Leaf: - Treatment of stomach and liver pains (drinkable decoction) - Hemostatic, on wounds (powder for external use) - Anti-venom, on snake and scorpion bites and stings (powder for external use) - In plasters, maturative and curative for boils and abscesses - Treatment of eczema and various dermatoses (powder for external use). Flower: - In baths and antiseptic lotions for infant hygiene (infusion) - Body care -Face care - Treatment of cracked breasts [30,56,58] |
Henné1: name of Lawsonia inermis leaf powder; lhmrira2: rock used in the preparation of traditional remedies; ghassul3: clay used in Morocco for rinsing hair and skin; miswak4: traditional toothbrush; khol5: mineral extracted from Atlas mountain rocks used for eye make-up.
The species studied belong to 19 families [Figure 3]. According to the monograph, various plant parts – such as leaves, aerial parts, roots, bark, stems, and seeds – are used to prepare traditional remedies in forms such as decoction, infusion, or powder. These remedies are employed to treat a range of ailments including digestive problems, skin infections, diabetes, cancer, rheumatism, oral and dental issues, liver problems, and others.
In this study, the Amaranthaceae and Fabaceae families were found to be widely used for treating digestive problems. Within the Amaranthaceae family, species from the genus Salsola, such as Salsola tetragona and Salsola tetrandra, are traditionally used to treat skin infections. Moreover, Atriplex halimus, another member of this family, is among the most commonly utilized plants due to its broad therapeutic applications, including managing diabetes and supporting oral hygiene.
In addition, A. raddiana, commonly called “telh” by the Sahrawi people, is traditionally prepared as a decoction and valued for its digestive benefits, pronounced hypoglycemic effects, and especially its role in managing obesity. Similarly, infusions prepared from the leaves of Senna italicaa (Fabaceae) are traditionally used as digestive remedies.
Moreover, according to the results of our investigation, the Anacardiaceae family is represented by a single species, R. tripartita, locally known as “jdari.” The leaves and bark of this species are widely used in decoction to treat digestive disorders. Indeed, the local population frequently uses numerous plant species to treat various skin infections such as pimples or burns by applying them as natural compresses. For example, the latex of L. arborescens (Asteraceae), commonly found throughout most of the study area, is widely used to treat skin conditions such as pimples and to aid in thorn removal. In addition, Heliotropium erosum from the Boraginaceae family is traditionally applied to manage abscesses, swelling, and burns.
The ethnobotanical survey further highlighted that Sahrawi communities make use of certain medicinal plants to combat infertility. Among these, A. hierochuntica (Brassicaceae), locally called “lkemcha,” is especially valued for this purpose. It is particularly employed to treat microbial infections of the uterus, which can affect fertility. Midwives specifically use it as a decoction made from the whole plant to facilitate childbirth and alleviate pain. Beyond its gynecological applications, A. hierochuntica is also used to treat rheumatism and joint pain in the region. In addition, Mesembryanthemum theurkauffii (Aizoaceae) is traditionally recommended for treating infertility issues.
Our ethnobotanical study revealed that the Sahrawi population relies on several local medicinal plants to address dental health issues. Notably, Maerua crassifolia (Capparaceae), locally known as “Atyle,” is widely used for oral care. Conventionally, its stem serves as a natural toothbrush, referred to as “Miswak,” which helps clean the teeth effectively. In addition, decoctions prepared from its leaves are employed to relieve toothache, demonstrating both curative and preventive roles in maintaining oral hygiene.
Beyond dental care, the Sahrawi show a strong interest in natural body care. For promoting hair growth, two species, M. crassifolia and Ziziphus lotus subsp. saharae (Rhamnaceae) are commonly used. The dried leaves of these plants are ground into powder and applied directly to the scalp.
Our investigation also identified Nitraria retusa (Nitrariaceae) as a plant with broad medicinal applications. The local communities use it to treat skin infections and digestive disorders. Moreover, decoctions made from its leaves are traditionally consumed to counteract food poisoning, a common issue, particularly among nomadic Sahrawi living in desert environments.
Finally, the study highlights the important role of Z. gaetulum, locally called “Laagaya,” in Saharan traditional medicine. This widely distributed desert plant is prized for its diverse therapeutic properties. Various parts, especially leaves and flowers, are used to manage skin infections and digestive problems. Notably, Z. gaetulum stands out as the sole species recognized for treating liver disorders and multi-organ diseases, underscoring its unique and vital place in the pharmacopoeia of the Saharan region.
3.2. Phytochemical study of the harvested plants
3.2.1. TLC analysis
The analysis of the methanolic extracts of the AMRs through TLC yielded a quantitative and semi-qualitative representation of the phenolic profiles of the various species [Figure 6].
 | Figure 6: NEU reagent-developed chromatoplates visualized under ultraviolet at 365 nm (Migration solvents: ethyl acetate – formic acid – acetic acid – water (100:11:11:27 v/v), support: silica on aluminum). 42: Maerua crassifolia; 35: Calotropis procera; 37: Acacia raddiana (fruit); 15: Anastatica hierochuntica; 14: Rhus tripartita; 13: Mesembryanthemum theurkauffii; 10: Ziziphus lotus; 8: Zygophyllum gaetulum; 3: Heliotropium erosum; 4: Atriplex halimus; 5: Launaea arborescens; 6: Salsola tetragona; 7: Salsola tetrandra; 9: Nitraria retusa; 12: Freesia corymbosa; 16: Trichodesma amplexicaule; 18: Sedum sediforme; 20: Deverra denudata; 21: Hyoscyamus muticus; 22: Farsetia occidentalis; 23: Limonium tuberculatum; 25: Lotus chazaliei; 27: Pergularia tomentosa; 29: Lycium intricatum; 31: Lotus corniculatus; 32: Spergularia diandra; 33: Senna italicaa; 34: Haloxylon scoparium; 36: Arthrocnemum macrostachyum; and 37: Acacia raddiana (leaf). [Click here to view] |
The identification of phenolic compounds was carried out based on standards of flavonoids and total phenols, through a reference TLC performed under the same experimental conditions and with the same solvent system. This reference TLC allowed us to confirm the fluorescence of certain compounds, as well as the distance of the bands from the application point, which facilitated their identification. This approach served as a reference to determine the presence of various phenolic compounds in our samples before conducting more detailed phytochemical analyses.
The chromatoplate results reveal distinct fluorescent bands for the studied species, indicating their abundance in phenolic compounds such as flavonoids and phenolic acids. This fluorescence varies significantly based on the compound type, particularly within the flavonoid group, specifically the flavonol subclass. For example, kaempferol displays a vivid yellow fluorescence, quercetin emits an orange-yellow fluorescence, and myricetin displays an orange-red fluorescence. Phenolic acids, such as chlorogenic acid, are identifiable by their blue fluorescence. The flavonoid derivatives, especially flavonols and kaempferol, were found in R. tripartita, L. arborescens, T. amplexicaulis, Deverra denudata, L. tuberculatum, Pergularia tomentosa, Lycium intricatum, Spergularia diandra, and S. italicaa.The presence of myricetin is noted in R.tripartita and the leaves of A. raddiana. The profile of H. erosum, L. arborescens, D. denudata, L. intricatum, and S. italicaa indicates the presence of phenolic acids, such as chlorogenic acid.
3.2.2. Determination of total phenols, total flavonoids, and the antioxidant activity
The methanolic extracts from different plants were assessed for their total phenols and total flavonoid content, alongside their antioxidant activity using the scavenger method of DPPH. The analyses were conducted in triplicates [Table 4].
Table 4: The results of the total flavonoids and total phenols assays in the harvested plants are expressed in µg/mg DM, while the antioxidant activity IC50 is expressed in μg/mL.
| Family | Scientific name | Used part | Total phenols (µg AG/mg) | Flavonoids (µg/mg) | Antioxidant activity IC50 (μg/mL) | |
|---|---|---|---|---|---|---|
| Anacardiaceae | 14 | Rhus tripartita Moffet | Leaf | 476±1.15a | 253.97±0.01a | 15.35±0.53s |
| Apocynaceae | 35 | Calotropis procera (Aiton) W.T.Aiton | Leaf | 35.4±0.40k | 26.83±0.04i | 83.49±0.68e |
| 27 | Pergularia tomentosa L. | 32.8±0.51k | 19.79±0.03m | 66.13±0.70h | ||
| Aizoaceae | 13 | Mesembryanthemum theurkauffii (Maire) Maire | Leaf | 45.8±0.17j | 4.61±0.10v | 48.35±0.80m |
| Amaranthaceae (Chenopodiaceae) | 4 | Atriplex halimus L. | Leaf and stem | 7.8±0.11m | 12.21±0.05q | 104.78±0.73b |
| 6 | Salsola tetragona Delile | Leaf | 3.8±0.17p | 65.14±0.03d | 100.41±0.92c | |
| 7 | Salsola tetrandra Forssk. | 2.85±0.31p | 2.77±0.01w | 78.64±0.73f | ||
| 34 | macrostachyumScoparium Pomel | Leaf and stem | 4±0.17p | 7.57±0.16st | 94.85±0.95d | |
| 36 | Arthrocnemum macrostachyum (Moric.) K.Koch | Leaf | 63.6±0.46h | 8.39±0.03r | 32.95±0.77op | |
| Asteraceae | 5 | Launaea arborescens (Batt.) Murb. | Stem | 99.2±0.23f | 68.29±0.01c | 29.96±0.70pq |
| Apiaceae | 20 | Deverra denudata (Viv.) Pfisterer & Podlech | 14.6±0.28n | 22.48±0.01k | 58.96±0.81i | |
| Boraginaceae | 3 | Heliotropium erosum Lehm. | Leaf | 52±0.23i | 14.01±0.01p | 32.74±0.67op |
| Brassicaceae | 22 | Farsetia occidentalis B.L.Burtt | Stem | 28.2±0.17l | 20.98±0.01l | 53.67±0.75kl |
| 15 | Anastatica hierochuntica L. | 10.3±0.17m | 1.88±0.04x | 71.44±0.79g | ||
| Crassulaceae | 18 | Sedum sediforme (Jacq.) Pau | Leaf | 46.4±0.28j | 13.71±0.05p | 41.98±0.84n |
| Capparaceae | 42 | Maerua crassifolia Forssk. | 27.5±0.23l | 7.03±0.01st | 49.45±0.93lm | |
| Caryophyllaceae | 32 | Spergularia diandra (Guss.) Boiss. | Leaf | 3±0.11p | 18.44±0.03n | 80.64±0.72ef |
| Fabaceae | 25 | Lotus chazaliei H.Boissieu | Leaf | 101.8±1.03f | 7.64±0.01s | 205.03±0.77a |
| 33 | Senna italicaa Mill. | 76.2±0.17g | 70.98±0.01b | 35.49±0.72n | ||
| 37 | Acacia raddiana Savi | Fruit | 62.1±0.05k | 5.07±0.04v | 19.66±0.89r | |
| 37’ | Acacia raddiana Savi | Leaf | 317±0.23b | 46.32±0.39f | 18.03±0.71rs | |
| 31 | Lotus corniculatus L. | Stem | 20.5±0.28m | 17.99±0.04n | 58.63±0.84ij | |
| Frankeniaceae | 12 | Frankenia corymbosa Desf. | Leaf and stem | 122±1.73e | 15.36±0.02o | 21.37±0.56r |
| Nitrariaceae | 9 | Nitraria retusa (Forssk.) Asch. | Leaf | 44.2±0.11j | 33.35±0.05g | 61.02±0.67i |
| Plumbaginaceae | 23 | Limonium tuberculatum (Boiss.) Kuntze | Leaf | 130±1.15d | 24.43±0.04j | 14.43±0.77s |
| Rhamnaceae | 10 | Ziziphus lotus subsp saharae (Batt. and Trab.) Maire | Leaf | 188±0.57c | 13.76±0.15p | 25.84±0.78q |
| Solanaceae | 21 | Hyoscyamus muticus L. | Leaf | 34±0.86k | 27.58±0.01h | 54.40±0.78jk |
| 29 | Lycium intricatum Boiss. | 74.8±0.46g | 63.49±0.04e | 29.95±0.54pq | ||
| Tamaricaceae | 16 | Tamarix amplexicaulis Ehrenb. | Leaf and stem | 60.8±0.46h | 6.57±0.01u | 20.89±0.57r |
| Zygophyllaceae | 8 | Zygophylum gaetulum Emb. and Maire | Leaf | 21.4±0.23m | 7.48±0.01st | 78.90±0.97f |
Data are presented as mean±Standard Error (n=3). Different letters indicate statistically significant differences according to Tukey’s post-hoc test (P<0.05).
A one-way ANOVA was conducted to assess differences in total phenolic content, flavonoid content, and antioxidant activity (IC50) among the 29 plant species analyzed. The results showed highly significant effects of species on all three parameters (phenolic content: F(28,58) = 24,485.85, P < 0.0001; flavonoid content: F(28,58) = 269,504.27, P < 0.0001; antioxidant activity: F(28,58) = 2,623.50, P < 0.0001), highlighting considerable variation between species. Tukey’s post hoc test identified homogeneous groups within each parameter; species sharing the same letter were not significantly different (P > 0.05), whereas those assigned different letters showed statistically significant differences. These findings indicate that differences in phytochemical content correspond to variations in antioxidant activity across the studied species.To explore the relationships between phytochemical contents and antioxidant activity, Pearson correlation analysis was performed. The results are presented in Table 5.
Table 5: Pearson correlation matrix between phenolic content, flavonoid content, and antioxidant activity (IC50).
| Parameters | Total phenols | Flavonoids | Antioxidant activity |
|---|---|---|---|
| Total phenols | 1 | 0.76** | −0.39** |
| Flavonoids | 0.76** | 1 | −0.26* |
| Antioxidant activity | −0.39** | −0.26* | 1 |
Total phenolic and flavonoid contents showed a strong positive correlation between them (r = 0.758, P < 0.0001), according to Pearson correlation analysis, suggesting that these compound classes often coexist within the species under study. On the other hand, phenolic content (r = −0.386, P = 0.00017) and flavonoid content (r = −0.258, P = 0.0139) were found to significantly correlate negatively with antioxidant activity (IC50). As indicated by lower IC50 values, the finding suggests that increased antioxidant capacity is correlated with higher concentrations of phenolics and flavonoids. Results for total flavonoids and total phenols showed a variation between the species.
Total phenol levels ranged from 3 μg/mg to 476 μg/mg. The highest content was observed in R. tripartita, recording 476 μg/mg, followed by A. raddiana leaves with a value of 317 μg/mg. L. intricatum, S. italicaa, L. arborescens, L. chazaliei, F. corymbosa, L. tuberculatum, and Z. lotus subsp. saharae also exhibited elevated levels of total phenols, ranging from 74.8 μg/mg to 188 μg/mg. However, other species such as L. corniculatus, Z. gaetulum, M. crassifolia, F. occidentalis, P. tomentosa, H. muticus, C. procera, N. retusa, M. theurkauffii, S. sediforme, H. erosum, T. amplexicaulis, A. raddiana (fruit), and Arthrocnemum macrostachyum show average levels ranging from 20.5 μg/mg to 63.6 μg/mg. On the other hand, D. denudata, A. hierochuntica, A. halimus, H. scoparium, S. tetragona, S. diandra, and S. tetrandra display relatively low phenol levels ranging between 14.6 μg/mg and 3 μg/mg. The lowest phenolic content was observed in Salsola diandra, with a value of 3 μg/mg.
Regarding total flavonoid content, values ranged from 1.88 μg/mg up to 253.97 μg/mg. The highest level was found in R. tripartita (253.97 μg/mg), followed by Salsola italicaa, L. arborescens, Salsola tetragona, Launaea intricatum, and Acacia raddiana (leaves), all exhibiting relatively high flavonoid contents ranging between 70.98 μg/mg and 46.32 μg/mg. The average total flavonoid levels ranged from 33.35 μg/mg to 12.21 μg/mg were recorded in N. retusa, H. muticus, C. procera, L. tuberculatum, D. denudata, F. occidentalis, P. tomentosa, S. diandra, L. corniculatus, F. corymbosa, H. erosum, S. sediforme, Z. lotus subsp saharae, and A. halimus. The low levels of flavonoid contents are exhibited by A. macrostachyum, L. chazaliei, Z. gaetulum, H. scoparium, M. crassifolia, T. amplexicaulis, A. raddiana (fruit), M. theurkauffii, S. tetrandra, and A. hierochuntica with values ranging from 8.39 μg/mg to 1.88 μg/mg, with the lowest content found in A. hierochuntica.
The analysis of the antioxidant potential of the different plants tested using the DPPH radical method reveals that the majority of plant extracts displayedsignificant antioxidant activity. The capacity of plant extracts to scavenge the DPPH radical ranged from 14.43 μg/mL to 205.03 μg/mL. Indeed, L. tuberculatum, R. tripartita, A. raddiana (leaf and fruit), T. amplexicaulis, F. corymbosa, and Z. lotus subsp. saharae exhibited the highest antioxidant power, with the IC50 values ranging from 14.43 to 25.84 μg/mL. The highest value was obtained with L. tuberculatum. However, the other species showed quite interesting antioxidant capacity, with IC50 values ranging from 29.95 to 66.13 μg/mL. These species include L. intricatum, L. arborescens, M. crassifolia, H. erosum, A. macrostachyum, M. theurkauffii, and N. retusa. Low levels of antioxidant capacity were revealed by A. hierochuntica, S. tetrandra, Z. gaetulum, S. diandra, C. procera, H. scoparium, S. tetragona, and A. halimus, with IC50 values ranging from 71.44 μg/mL to 104.78 μg/mg. The lowest antioxidant capacity was observed in L. chazaliei H. Boissieu, with an IC50 value of 205.03 μg/mL.
4. DISCUSSION
Our investigation revealed the prevalence of the Amaranthaceae (Chenopodiaceae), Fabaceae and Asteraceae families which are well-known for their significance in the Saharan ecosystem [16]. The Amaranthaceae family plays a crucial role in the local diet, offering protein-rich edible seeds [59]. In addition, certain Salsola species within this family have been reported in the literature for their notable antioxidant and anti-inflammatory activities [60]. Locally, a decoction of the leaves is employed to address gastric problems and skin infections, as documented by Bellakhdar [30]. Among all the species studied in this research, we observed that A. halimus and H. scoparium, halophytes known for their diverse therapeutic uses, notably in the treatment of cancer, diabetes, gastric problems, as an emetic, and for mouth and teeth care, as previously reported [30,32,34,35]. In addition, these species demonstrate antidiarrheal and anti-inflammatory properties [31].
The Fabaceae family renowned for its contribution to soil nitrogen fixation and natural fertilization [61] is primarily characterized by the prevalence of Acacias, the gum obtained from Acacia trees is commonly utilized by the local population due to its hypoglycemic effect and its efficacy in treating digestive disorders. During our survey, we noted that the Acacia gum employed as as tea and in decoctions, often in combination with Eucalyptus resin, for the treatment of skin infections. These traditional uses are corroborated by several authors [30,48-50].
The Asteraceae family, recognized for its significant diversity in terms of species, is predominantly represented by Launaea sp. In addition to its therapeutic properties, L. arborescens, known locally as Oum lbina, is utilized for treating skin infections and removing thorns. Moreover, it serves as a source of food for animals [30].
Due to the harsh conditions of the desert, locals are more vulnerable to snake and scorpion attacks. Consequently, local populations frequently use many plant species as antidotes or antivenoms, commonly prepared as decoctions or poultices. These include H. scoparium, Cratolaria saharae, Z. gaetulum [30], H. erosum, and E. officinarum [30,32,62,63]. In addition, infusions made from leaves and whole plant of certain species, such as L. arborescens and C. procera, are used as vermifuges [30,37,38].
Our investigation revealed that leaves are the most commonly used parts, probalbly due to their abundance in various metabolites, such as phenolic compounds. These compounds have attracted considerable attention due to their antioxidant properties, which enable them to neutralize free radicals and reduce oxidative damage within cells [64-67]. By combating oxidative stress, antioxidants play an important role in preventing a range of diseases, including cardiovascular conditions, neurodegenerative disorders, certain cancers, and other ailments affecting the intestines, skin, and respiratory system.
The survey conducted in this study revealed that the majority of traditional remedies used by the local population involve decoctions, aligning with findings from other reports [68,69]. This preparation method is widely practiced among Saharan communities to treat digestive ailments such as stomachaches, intestinal ulcers, and bloating. Many plants are regarded as effective against these digestive problems, including the leaves of A. halimus and both the leaves and bark of R. tripartita, as previously documented by several authors [16,30].
The survey also indicated that species such as Salsola spp., L. arborescens, H. erosum, and Ziziphus lotus subsp. saharae are commonly used to treat skin infections, which is consistent with results reported in numerous similar studies [30,32,33,52-54,62,63].
We also noted that for their oral hygiene the local population, frequently use traditional toothbrushes made from M. crassifolia wood, locally known as “Atyle.” This traditional practice is also documented by Bellakhdar [30]. A. halimus leaves are also employed for similar purposes. Furthermore, the leaves of Z. lotus Subsp saharae have been shown to have an effect on abscesses, as confirmed by several previous reports [30,52-54]. According to the Bencheikh et al. [54], the leaves and fruits are ground into powder, mixed with sour milk or water, and then applied as poultices to abscesses.
The monograph also mentions new uses for some species and traditional recipes, such as the use of M. theurkauffii and A. raddiana as fat-burning plants, and the use of A. hierochuntica to treat rheumatism, with a precise recipe included. In addition, the traditional uses of some species, such as M. theurkauffii and H. erosum, are poorly studied. In this study, we have collected species that are not well known or previously studied, and we have evaluated their phytochemical composition. These species include F. occidentalis, F. corymbosa, D. denudata, L. chazaliei, and L. tuberculatum.
The chromatography results showed that the majority of the harvested species are rich in phenolic compounds, notably R. tripartita, L. arborescens, T. amplexicaulis, D. denudata, L.tuberculatum, P.tomentosa, L.intricatum, S.diandra and S.italicaa, H.erosum, L.arborescens, D.denudata, L.intricatum, and S. italicaa. Phenolic compounds are well recognized for their antioxidant [70] and anti-inflammatory [71] activities, along with various other health benefits. The Saharawi population extensively uses R. tripartita and Salsola italicaa to manage digestive disorders, whereas L. arborescens is particularly valued for its effectiveness against skin infections. Previous research on R. tripartita has highlighted its high phenolic content and remarkable antioxidant capacity [72,73]. Another study on S. italicaa extract revealed its richness in total phenols, flavones, tannins, alkaloids, and quinone derivatives [74], while another study on L. arborescens revealedits abundance of flavonoids, tannins, coumarins, and alkaloids [75]. The phenolic acids, flavonoids, and tannins are also the primary phytochemical compounds in the Tamarix genus [76], which is also aligned with the results obtained for T. amplexicaulis Ehrenb. In addition, D. denudata also demonstrated richness in phenolic compounds. The Deverra genus renowned for its biological activities, attributed to the presence of essential oils, coumarins, furocoumarins, flavonoids, and phenolic compounds [77]. L. intricatum also exhibited a high phenolic content, consistent with the results reported by Bendjedou [78]. This finding suggests that the species is a promising source of bioactive compounds, particularly phenolics, with potential pharmaceutical and biomedical applications.
The plants studied originate from the Sahara Desert, where they face extreme environmental stresses such as intense heat, water scarcity, arid soils, and drastic climatic fluctuations. These harsh conditions trigger adaptive responses, leading to increased phenolic compound production, which contributes to their strong antioxidant capacities. This adaptation enables these plants to survive and thrive under challenging conditions. For example, R. tripartita exhibits the highest phenolic content along with significant DPPH radical scavenging activity. Our results (IC50 = 15.35 μg/mL) closely match those reported by Mohammed [79] (IC50 = 15.64 μg/mL) and Itidel et al. [80] (IC50 = 16 μg/mL). Phenolic profiling identified compounds such as gallocatechin, quercetin, myricetin, and kaempferol, among others [79,80]. The abundance of these phenols supports the traditional use of various parts of R. tripartita in Saharan medicine to relieve stomachaches and treat digestive disorders. Numerous recent studies further confirm the significant antioxidant potential of this species [72,73,81,82].
Regarding the Fabaceae family, our findings on Acacia leaf extracts revealed a high phenolic content and antioxidant capacity, in agreement with several previous reports [83-86]. However, some authors [49] previously reported a higher antioxidant capacity compared to our study, although the total phenol content was similar to our obtained values. This could explain the traditional use of A. raddiana in the treatment of several diseases, including digestive problems and diabetes. The S. italicaa belonging to the Fabaceae family exhibited very similar results. The Senna genus, in general, has been previously reported to be rich in secondary metabolites and to have a high antioxidant activity [87,88]. Apreviously conducted phytochemical screening revealed the presence of some important secondary metabolites, such as anthocyanins, leucoanthocyanins, catechins, tannins, and flavonoids [89]. The results of the phytochemical screening conducted by Dabai et al. [90] further emphasize the pharmacological importance of S. italicaa, justifying its use in the traditional medicine, especially the leaves, as a laxative, purgative, and anti-icteric, as also revealed in our survey among traditional herbalists. A study by Towanou et al. [74] reported that both methanolic and aqueous extracts of S. italicaa leaves exhibit free radical scavenging activity, antioxidant properties, and contain various secondary metabolites. Similarly, research on Z. lotus subsp. saharae has highlighted its promising antioxidant potential [52], which is consistent with our findings. In addition, the phenolic compound contents obtained in our study are higher than those reported by some authors [91,92], which explains the significant traditional use of Z. lotus as a remedy to treat skin problems and diabetes.
Our results indicate that L. intricatum also possesses high level of phenolic compounds and a strong antioxidant capacity. The Lycium genus (Solanaceae family) is recognized for its abundance of antioxidant compounds [93]. There are no sufficient data on leaves of L. intricatum as most studies have primarily focused on the fruits. The contents of total flavonoid (63.49 μg/mg) and total phenols (74.8 μg/mg) recorded in our study exceed those reported by a previous study [94], which found 28.91 μg/mg and 50.93 μg/mg for total flavonoids and total phenols, respectively.
L. tuberculatum has the highest antioxidant capacity among the plants studied and exhibited also a high content of total phenols and total flavonoids. However, the phytochemical profile of this plant remains insufficiently explored. A study conducted by Kennouche and Bentamene [95] assessed the antioxidant capacity of the ethyl acetate extract of Limonium pruinosum using the DPPH assay. The extract exhibited notable antioxidant activity, indicating a significant presence of antioxidant compounds within the Limonium genus. Moreover, several species from this genus have demonstrated antimicrobial activity, suggesting their potential application in the treatment of specific infections [96]. Diuretic properties have also been reported in some Limonium species [32].
The species belonging to Asteraceae family, including the genus Launaea, are known for its wealth in triterpenoids, flavonoids and phenolic compounds contributing to their anti-inflammatory, anti-hyperlipidemic, hepatoprotective, antioxidant, and cytoprotective activities. They provide significant protection against cardiovascular disease and certain types of cancer [38]. L. arborescens reveals notable levels of phenolic compounds consistent with findings reported by Belhi and Cheriti [75], thus supporting its various traditional uses mentioned in our survey, including the treatment of skin infections and its use as a vermifuge. Species of the Launaea genus extensively utilized in the traditional folk medicine across regions where they are distributed [97,98].
In our study, F. corymbosa (Frankeniaceae) showed elevated concentrations of phenolic compounds and significant antioxidant potential, despite limited data on its phytochemical profile. Significantly, although previous studies on different Frankenia species indicated lower phenolic levels [99], one investigation documented a total phenol content of 172.65 mg GAE/g [100], which is very close to the observed value (122 μg/mg). The antioxidant potential of the genus was confirmed by Mennai et al. [101], who documented an IC50 of 9.35 μg/mL, in contrast to our value of 21.37 μg/mL. Conversely, the two studied Apocynaceae species, C. procera and P. tomentosa, exhibited moderate levels of total phenols and flavonoids. The phenolic content of C. procera (35.4 μg GAE/mg) exceeded that documented by Nagda et al. [102] (9.16 μg/mg) but was inferior to the 71.32 mg GAE/g reported by Ahmed et al. [103].
In contrast to another study [104], which reported an IC50 value of 28.57 μg/mL, our study’s antioxidant capacity of C. procera (IC50 = 83.49 μg/mL) was lower. Alghanem and El-Amier [105] found several classes of secondary metabolites in P. tomentosa, including tannins, flavonoids, saponins, glucosides, and steroids. These results are consistent with our screening for phytochemicals. However, their study also reported a significantly higher antioxidant capacity than that observed in our analysis. This discrepancy may be due to differences in extraction solvents, plant material origin, or the specific part of the plant analyzed, underlining the influence of methodological variations on antioxidant evaluation.
H. erosum (Boraginaceae family) demonstrated moderate phenolic compound levels, but displayed an interesting IC50. This study demonstrated, similar to our findings, a moderate total phenol content (42.3 mg GAE/g), which is close to the values obtained in our study (52 μg/mg), but exhibited a low antioxidant capacity (430 μg/mL) compared to the value that we obtained (32.4 μg/mL) [84,106-108].
Regarding S. sediforme (Crassulaceae family), previous studies on species within the same genus reported higher total phenolcontents (136.9 mg AG/g and 113.3 mg AG/g, respectively), along with higher total flavonoids. However, these studies found lower antioxidant capacity compared to the results obtained in our study [109,110].
According to our ethnobotanical survey, M. crassifolia (Capparaceae family) is widely used in traditional medicine by the local population, particularly for addressing digestive issues and maintaining oral hygiene. Indeed, various parts of the plant could be utilized and exhibit different biological activities [30]. Its phenolic content is moderate, but its antioxidant capacity is substantial. Findings were juxtaposed with those of Pure [111], who reported lower values of total flavonoids of 17.49 mg/100 g and total phenols of 27.29 mg/100 g, figures inferior to those revealed in our study, with total flavonoids value of 7.03 μg/mg and total phenol value of 27.5 μg/mg. The antioxidant capacity obtained in our study (IC50 = 49.45) surpasses that found in other study, who reported an IC50 value of 448 μg/mL [112].
The leaf extract of N. retusa (Nitrariaceae family) exhibits a moderate phenolic content along with a moderate antioxidant capacity. Conventionally, the leaves of this plant are extensively utilized in Saharan remedies to address diverse health issues, including digestive problems and poisoning. In our study, the total phenolic and flavonoid contents were comparable to those reported by Mounira et al. [113], who found 17.56 mg AG/g of phenols and 42.59 mg R/g of flavonoids. On the other hand, the antioxidant activity reported in that study was stronger, with an IC50 of 1.64 μg/mL, which is lower than the value observed in our results.
T. amplexicaulis (Tamaricaceae) showed moderate levels of phenolic compounds but exhibited strong antioxidant activity. According to our ethnobotanical data, this genus is traditionally used to treat gastrointestinal disorders, wounds, diabetes, and dental issues, which may be attributed to the presence of phenolic acids, flavonoids, and tannins. Several preclinical studies have reported various biological activities in Tamarix species, including anti-diabetic, hepatoprotective, wound-healing, and anti-inflammatory effects [76]. Our study’s findings surpass those reported for Tamarix aphylla in other research [114].
The Zygophyllaceae family is renowned for its abundance of specific alkaloids, compounds recognized for their biological effects, particularly in Peganum harmala seeds. These seeds possess hallucinogenic properties and contain a natural dye formerly utilized for carpet dyeing. P. harmala, classified as a hallucinogenic plant, is known for its richness in harmine and harmanol alkaloids, which are both used in traditional and conventional medicine [115]. Z. gaetulum, widely distributed in the Saharan desert, has attracted growing interest due to its remarkable biological activities. Locally, it is traditionally used to treat skin infections, digestive problems, and liver disorders. In one study, this species showed a low antioxidant activity (IC50 = 200 μg/mL), which contrasts with our results, where a stronger activity was observed (IC50 = 78.9 μg/mL) [116].
The present study revealed significant differences among the studied species in terms of total phenolic content, flavonoid content, and antioxidant activity (IC50), as demonstrated by one-way ANOVA (P < 0.0001). Tukey’s post hoc test allowed the classification of species into distinct groups, showing that some plants had notably higher levels of phytochemicals and antioxidant potential than others. These differences reflect the natural variability in secondary metabolite production among plant species, which is influenced by both genetic makeup and environmental conditions. In addition, Pearson correlation analysis showed a strong positive correlation between total phenolic and flavonoid contents (r = 0.76, P < 0.0001), suggesting that flavonoids make up a substantial portion of the total phenolics. A significant negative correlation was also observed between antioxidant activity and both phenolic and flavonoid contents, indicating that species richer in these compounds tend to have stronger antioxidant effects (i.e., lower IC50 values). This relationship highlights the important role of phenolics and flavonoids in the radical scavenging activity of plant extracts. Overall, these results are in line with previous studies reporting similar associations between phenolic content, flavonoids, and antioxidant activity [118-121]. They support the idea that variability in these phytochemicals contributes to differences in antioxidant capacity, and they underline the relevance of these compounds as potential bioactive markers. Further chemical and pharmacological investigations on the most promising species are therefore encouraged.
Although this study mainly focused on flavonoids and phenolic acids, known for their antioxidant and therapeutic properties [117,119], it’s worth noting that other bioactive compounds, beyond antioxidants, may also play an important role in the observed medicinal effects. In reality, medicinal plants often contain a wide mix of active substances that can work together in a synergistic way, leading to more complex and enhanced pharmacological effects.
Alkaloids, terpenoids, and essential oils are some of the bioactive compounds that have been extensively studied for their pharmacological properties. Alkaloids, for instance, are well-known for their anticancer, analgesic, and antispasmodic effects. Medicinal plants contain a variety of bioactive compounds with important therapeutic roles. For instance, alkaloids present in some species are traditionally used to manage conditions such as cancer, chronic pain, and digestive problems. Terpenoids, meanwhile, are known for their anti-inflammatory, antiviral, and antimicrobial effects, making them valuable in treating infections and immune-related disorders. In addition, essential oils extracted from certain plants offer a range of benefits, including antiviral, antimicrobial, antioxidant, and relaxing properties, which can help alleviate various symptoms [120].
In addition to these compounds, many medicinal plants contain saponins, glycosides, and coumarins, which play important roles in modulating complex biological processes and contribute to their therapeutic effects. For example, saponins are known for their ability to lower cholesterol and regulate the immune system, glycosides have effects on heart function and blood sugar control, and coumarins are valued for their anticoagulant and anti-inflammatory properties.
The plants studied in this research contain a variety of bioactive molecules in addition to flavonoids and phenolic acids. For example, R. tripartita is rich in triterpenoids, which provide anti-inflammatory and anticancer properties, in addition to its antioxidant power [121]. The anthraquinones found in S. italicaa are responsible for its laxative and antimicrobial activities, while the flavonoids and saponins present in this plant play a key role in its antioxidant and anti-inflammatory effects. Similarly, A. raddiana contains flavonoids, tannins, and saponins that participate in its antimicrobial, hypoglycemic, and antioxidant properties. These reports underscore the importance of investigating various classes of bioactive compounds to deepen our understanding of the therapeutic potential of these plants and to guide future pharmacological studies.
In summary, the plants studied show considerable variation in their phenolic compound content, highlighting their diverse abilities to accumulate these important molecules. Differences observed in total phenol and flavonoid levels compared to previous reports can be explained by multiple factors. These include species differences, the locations and timing of harvest, and geoclimatic influences such as temperature, rainfall, and soil characteristics. In addition, methodological variations, such as the plant material used, sample preparation including drying and extraction methods, choice of solvents, assay techniques, and the particular plant parts analyzed also play a significant role. Interestingly, some species with high phenolic content displayed relatively low antioxidant activity, suggesting that antioxidant effects might arise from alternative pathways or mechanisms, including assays like 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid), ferric reducing antioxidant power, or β-carotene bleaching. Given these complexities, it is essential to consider all these variables when assessing phenolic content and antioxidant capacity, to better understand the therapeutic potential and traditional uses of these plants. Further research is necessary to clarify these relationships and to establish more precise links between phenolic compounds, antioxidant activities, and biological effects in the species studied.
5. LIMITATIONS AND FUTURE PERSPECTIVES
Phytochemical screening and TLC analysis provided preliminary information on the presence of phenolic compounds and flavonoids in the plants studied. However, the lack of confirmation by advanced instrumental methods such as liquid chromatography-mass spectrometry or high-performance liquid chromatography is a limitation of this study. Looking ahead, future research should focus on the isolation and advanced structural characterization of the main bioactive compounds. In addition, in vivo studies will be necessary to confirm the biological activity and safety of these plant extracts, particularly their antioxidant, anti-inflammatory, and antimicrobial properties. Understanding the mechanisms of action and assessing potential toxicity appear essential for the development of effective phytotherapeutic agents.
6. CONCLUSION
The study carried out in the Dakhla-Oued Ed-Dahab region revealed a rich floristic diversity and highlighted the extensive use of AMPs by the Sahrawi population. Among the 29 species identified, representing 19 botanical families most are spontaneous and endemic, with Amaranthaceae and Fabaceae being the most represented. Ethnobotanical data indicate that these plants are primarily used in traditional medicine to address a range of health issues, notably digestive disorders (25.71%), skin infections (20%), and diabetes (11.43%). The leaves are the most commonly used plant part (53.85%), and decoctions represent the predominant mode of preparation (63.16%). Phytochemical analyses confirmed the presence of significant amounts of phenolic compounds, particularly flavonoids and phenolic acids, which are closely associated with strong antioxidant activity. These findings highlight the therapeutic potential of the studied species, as the antioxidant properties of their bioactive compounds may contribute to the treatment of various ailments. A clear correlation was observed between phytochemical richness and biological efficacy, supporting the traditional use of these plants in local medicine. Noteworthy, examples include R. tripartita, A. raddiana, and Limonium tuberculatum, which exhibited high levels of bioactive compounds and marked antioxidant capacity. This work underscores the importance of ethnobotanical studies in promoting the value of southern Morocco’s flora and identifying promising natural resources for pharmaceutical development. By integrating traditional knowledge with phytochemical analysis, the study opens avenues for further exploration of the therapeutic potential of these underexplored species. It also emphasizes the need for sustainable and regulated use of this valuable plant heritage, ensuring its conservation and its responsible exploitation for future pharmacological applications.
7. ACKNOWLEDGMENTS
The authors would like to warmly thank Professor Mohamed FENNANE for his important and valuable help.
8. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
9. FUNDING
This research was supported by the National Center for Scientific and Technical Research (CNRST), Morocco (Grant No. PMA3-2021/15), Ibn Zohr University, and the National Agency for Medicinal and Aromatic Plants (ANPMA), Morocco.
10. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
11. ETHICAL APPROVALS
This study did not involve humans or animals and required no ethical approval.
12. DATA AVAILABILITY STATEMENT
All data supporting the findings of this study are included within the article.
13. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
14. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declare that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Cook KH, Vizy EK. Detection and analysis of an amplified warming of the Sahara Desert. J Clim. 2015;28(16):6560-80.[CrossRef]
2. Remini B. The sahar:A wind dynamics on surface and water in depth. Larhyss J. 2021;18(3):27-45.
3. Farr B, Farr WM, Cowan NB, Haggard HM, Robinson T. Exocartographer:A Bayesian framework for mapping exoplanets in reflected light. Astron J. 2018;156(4):146.[CrossRef]
4. Agoubi B. A review:Saltwater intrusion in North Africa's coastal areas-current state and future challenges. Environ Sci Pollut Res. 2021;28(14):17029-43.[CrossRef]
5. Lelieveld J, Proestos Y, Hadjinicolaou P, Tanarhte M, Tyrlis E, Zittis G. Strongly increasing heat extremes in the Middle East and North Africa (MENA) in the 21st century. Clim Change. 2016;137(1):245-60.[CrossRef]
6. Pastore G, Baird T, Vermeesch P, Bristow C, Resentini A, Garzanti E. Provenance and recycling of Sahara desert sand. Earth Sci Rev. 2021;216:103606.[CrossRef]
7. Bradai L, Bouallala M, Bouziane NF, Zaoui S, Neffar S, Chenchouni H. An appraisal of eremophyte diversity and plant traits in a rocky desert of the Sahara. Folia Geobot. 2015;50:239-52.[CrossRef]
8. Chehma A. Catalogue des Plantes Spontanées du Sahara Septentrional Algérien. Européenne:Éditions Universitaires Européennes;2019. 156.
9. Benkhaled A, Senator A, Boudjelal A, Kheniche A, Belbahi A, Reggami Y, et al. Oral acute toxicity and red blood cytotoxicity of the medicinal halophyte Limoniastrum guyonianum leaf extract. Farmacia. 2020;68(6):1136-46.[CrossRef]
10. Andersen GL, Krzywinski K, Gjessing HK, Pierce RH. Seed viability and germination success ofAcacia tortilis along land-use and aridity gradients in the Eastern Sahara. Ecol Evol. 2016;6(1):256-66.[CrossRef]
11. Malam Boukar AK, Yamba B, Lebailly P. Système D'exploitation et Potentialités Economiques des Cuvettes Oasiennes du Sud-est du Niger. In:Colloque Scientifique International Sur La Préservation et L'utilisation Durables des Systèmes Oasiens. Niamey, Niger;2016.
12. Damtie D, Mekonnen Y. Toxicity and oviposition deterrent activities of thyme essential oils against Anopheles arabiensis. Psyche A J Entomol. 2021;2021:1-7.[CrossRef]
13. Taha D, Bourais I, El Hajjaji S, Bouyahya A, Khamar H, Iba N. Traditional medicine knowledge of medicinal plants used in Laayoune Boujdour Sakia El Hamra region, Morocco. J Herbs Spices Med Plants. 2022;28(2):351-69.[CrossRef]
14. Alaoui MSB, Satrani B, Boussoula E, Ghanmi M. Etude ethnobotanique des plantes médicinales utilisées dans les provinces du sahara marocain. Int J Innov Appl Stud. 2018;24(2):789-801.
15. Mechaala S, Bouatrous Y, Adouane S. Traditional knowledge and diversity of wild medicinal plants in El Kantara's area (Algerian Sahara gate):An ethnobotany survey. Acta Ecol Sin. 2022;42(1):33-45.[CrossRef]
16. Idm'hand E, Msanda F, Cherifi K. Ethnobotanical study and biodiversity of medicinal plants used in the Tarfaya Province, Morocco. Acta Ecol Sin. 2020;40(2):134-44.[CrossRef]
17. El-Ghazouani F, El-Ouahmani N, Teixidor-Toneu I, Yacoubi B, Zekhnini A. A survey of medicinal plants used in traditional medicine by women and herbalists from the city of Agadir, southwest of Morocco. Eur J Integr Med. 2021;42:101284.[CrossRef]
18. Ouhaddou HOH, Alaoui A, Sezgin A. Ethnobotanical survey of medicinal plants used for treating diabetes in Agadir Ida Outanane region, Southwestern Morocco. Arab J Med Aromat Plants. 2020;6(2):72-86.
19. Eddouks M, Ajebli M, Hebi M. Ethnopharmacological survey of medicinal plants used in Daraa-Tafilalet region (Province of Errachidia), Morocco. J Ethnopharmacol. 2017;198:516-30.[CrossRef]
20. Aslaou F, Chafik K, Laarbi BM, Aitlhaj-Mhand R, El Hairach D, Ahid S, et al. The tragic complications due to abusive co-exposure to the traditional herbal based fattening recipes and dexamethasone in the Oued-Eddahab Region of Southern Morocco. J Xi'an Univ Archit Technol. 2023;14(7):573-84.
21. Ullah A, Munir S, Badshah SL, Khan N, Ghani L, Poulson BG, et al. Important flavonoids and their role as a therapeutic agent. Molecules. 2020;25(22):5243.[CrossRef]
22. Kabran GRM, Ambeu NC, Mamyrbékova-Békro JA, Békro YA. Total phenols and flavonoids in organic extracts from ten plants used in traditional therapy for breast cancer in Côte d'Ivoire. Eur J Sci Res. 2012;68(2):182-90.
23. Neu R. Ein neues Reagenz zum Nachweis und zur Unterscheidung von flavonen im papierchromatogramm. Naturwissenschaften. 1956;43(4):82.[CrossRef]
24. Andary C. Documentation Chimique et Pharmaceutique Pour l'AMM du MERALOPS Comprimés. Monaco, France:Lab Allergan-Dulcis;1990.
25. El Hariri B, SalléG, Andary C. Involvement of flavonoids in the resistance of two poplar cultivars to mistletoe (Viscum album L.). Protoplasma. 1991;162(1):20-6.[CrossRef]
26. Amri O. Phytochimie et Activités Biologiques des Extraits Phénoliques et Lipidiques de Pistacia atlantica dans Trois Régions du Maroc:Activités Anti-inflammatoires, Anti-oxydantes et Activitésur des Cultures Cellulaires. Morocco:Ibn Zohr Agadir;2018.
27. Lekbir A, Sebsis S, Bouhbila A. Contribution àL'étude Des Métabolites Secondaires des Feuilles de la Plante Jujubier Zizyphus lotus L. Leurs Effets Biologiques. Algeria:Universitéde Larbi Ben M'hidi-Oum El Bouaghi;2022.
28. Masuda T, Yonemori S, Oyama Y, Takeda Y, Tanaka T, Andoh T, et al. Evaluation of the antioxidant activity of environmental plants: Activity of the leaf extracts from seashore plants. J Agric Food Chem. 1999 Apr 1;47(4):1749-54.[CrossRef]
29. Loo AY, Jain K, Darah I. Antioxidant activity of compounds isolated from the pyroligneous acid, Rhizophora apiculata. Food Chem. 2008;107(3):1151-60.[CrossRef]
30. Bellakhdar J. In:Fennec L, editor. La Pharmacopée Marocaine Traditionnelle - Médecine Arabe Ancienne et Savoirs Populaires. 2nd ed. French:LE FENNEC EDIT;2020. 1-1370.
31. Dehimi K, Djoudi Z, Damamna S, Boulaouad A, Maadadi AR, Khennouf S. A contribution to the valorization of two medicinal plants:Atriplex halimus Sub. Sp. Schweinfurthii and Bunium incrassatum, growing in the region of M'sila (North-East Algeria). Indian J Nov Drug Deliv. 2021;12(4):208-16.
32. Afrokh M, Tahrouch S, Elmehrach K, Fahmi F. Ethnobotanical, phytochemical and antioxidant study of fifty aromatic and medicinal plants. Chem Data Collect. 2023;43:100984.[CrossRef]
33. ElNaggar MH, Eldehna WM, Abourehab MAS, Abdel Bar FM. The old world Salsola as a source of valuable secondary metabolites endowed with diverse pharmacological activities:A review. J Enzyme Inhib Med Chem. 2022;37(1):2036-62.[CrossRef]
34. Kharchoufa L, Bouhrim M, Bencheikh N, El Assri S, Amirou A, Yamani A, et al. Acute and subacute toxicity studies of the aqueous extract from Haloxylon scoparium Pomel (Hammada scoparia (Pomel)) by oral administration in rodents. Biomed Res Int. 2020;2020:4020647.[CrossRef]
35. Benkherara S, Bordjiba O, Harrat S, Djahra AB. Antidiabetic potential and chemical constituents of Haloxylon scoparium aerial part, an endemic plant from Southeastern Algeria. Int J Second Metab. 2021;8(4):398-413.[CrossRef]
36. ElNaker NA, Yousef AF, Yousef LF. A review of Arthrocnemum (Arthrocaulon) macrostachyum chemical content and bioactivity. Phytochem Rev. 2020;19(6):1427-48.[CrossRef]
37. Batool H, Hussain M, Hameed M, Ahmad R. A review on Calotropis procera its phytochemistry and traditional uses. Big Data Agric. 2020;2(2):56-8.[CrossRef]
38. Cheriti A, Belboukhari M, Belboukhari N, Djeradi H. Phytochemical and biological studies on Launaea Cass. (Asteraceae) from Algerian Sahara. Curr Top Phytochem. 2012;11:68-78.
39. Sekkoum K, Belboukhari N, Cheriti A. New flavonoids from bioactive extract of Algerian medicinal plant Launeae arborescens. Asian Pac J Trop Biomed. 2014;4(4):267-71.[CrossRef]
40. Seddiki LS, Belboukhari N, Ould el Hadj-khelil A, Sulaiman MR, Sekkoum K, Cheriti A. Pharmacological studies on aqueous extract of Launaea arborescens from southwest Algeria. J Ethnopharmacol. 2021;275:114137. https://doi.org/10.1016/j.jep.2021.114137[CrossRef]
41. Abou-Elella F, Hanafy EA, Gavamukulya Y. Determination of antioxidant and anti-inflammatory activities, as well as in vitro cytotoxic activities of extracts of Anastatica hierochuntica (Kaff Maryam) against HeLa cell lines. J Med Plants Res. 2016;10(7):77-87.[CrossRef]
42. Zin SR, Mohamed Z, Alshawsh MA, Wong WF, Kassim NM. Mutagenicity evaluation of Anastatica hierochuntica L. extract in vitro and in vivo. Exp Biol Med. 2018;243(4):375-85.[CrossRef]
43. Almundarij TI, Alharbi YM, Abdel-Rahman HA, Barakat H. Antioxidant activity, phenolic profile, and nephroprotective potential of Anastatica hierochuntica ethanolic and aqueous extracts against ccl4-induced nephrotoxicity in rats. Nutrients. 2021;13(9):29-73.[CrossRef]
44. El-Garawani IM, Abd El-Gaber AS, Algamdi NA, Saeed A, Zhao C, Khattab OM,et al. In vitro induction of apoptosis in isolated acute Myeloid Leukemia Cells:The role of Anastatica hierochuntica methanolic extract. Metabolites. 2022;12(9):878.[CrossRef]
45. Ckilaka KC, Akuodor GC, Akpan JL, Ogiji ED, Eze CO, Ezeokpo BC. Antibacterial and antioxidant activities of methanolic leaf extract of Maerua crassifolia. J Appl Pharm Sci. 2015;5(10):147-50.[CrossRef]
46. Adjou E, Koudoro Y, Sohounhloue DKC, Adjou ES, Koudoro AY, Nonviho G,et al. Phytochemical profile and potential pharmacological properties of leaves extract of Senna italicaa Mill. Am J Pharmacol Sci. 2021;9(1):36-9.
47. Towanou R, Konmy B, Yovo M, Dansou CC, Dougnon V, Loko FS, et al. Phytochemical screening, antioxidant activity, and acute toxicity evaluation of Senna italicaa extract used in traditional medicine. J Toxicol. 2023;2023:6405415.[CrossRef]
48. Geneviève Z, Adama K, BaléB, Hamidou TH, Gaston BAM, Vincent N, et al. Botanical and ethnoveterinary surveys of two acacias (Acacia raddiana and Acacia nilotica) exploited in small ruminant rearing in Sahelian area of Burkina Faso. Anim Vet Sci. 2017;5(5):63-8.[CrossRef]
49. Ramde-Tiendrebeogo A, Koala M, Ouattara N, Lompo M, Guisson IP. A comparative study of phytochemical profile and antioxidant activity of Sahelian plants used in the treatment of infectious diseases in northern part of Burkina Faso:Acacia seyalDelile and Acacia tortilis (Forssk.) Hayne subsp. raddiana (Savi). J Pharmacogn Phyther. 2019;11(3):74-9.[CrossRef]
50. Hnini M, Taha K, Aurag J. Botany, associated microbiota, traditional medicinal uses, and phytochemistry of Vachellia tortilis subsp. raddiana (Savi):A systematic review. J Agric Food Res. 2023;12:100566.[CrossRef]
51. Zar Kalai F, Dakhlaoui S, Hammami M, Mkadmini K, Ksouri R, Isoda H. Phenolic compounds and biological activities of different organs from aerial part of Nitraria retusa (Forssk.) Asch.:Effects of solvents. Int J Food Prop. 2022;25(1):1524-38.[CrossRef]
52. Abdoul-Azize S. Potential benefits of jujube (Zizyphus Lotus L.) bioactive compounds for nutrition and health. J Nutr Metab. 2016;2016:1-13.[CrossRef]
53. Marmouzi I, Kharbach M, El Jemli M, Bouyahya A, Cherrah Y, Bouklouze A, et al. Antidiabetic, dermatoprotective, antioxidant and chemical functionalities in Zizyphus lotus leaves and fruits. Ind Crops Prod. 2019;132:134-9.[CrossRef]
54. Bencheikh N, Radi FZ, Fakchich J, Elbouzidi A, Ouahhoud S, Ouasti M, et al. Ethnobotanical, phytochemical, toxicological, and pharmacological properties of Ziziphus lotus (L.) Lam.:A comprehensive review. Pharmaceuticals (Basel). 2023;16(4):1-575.[CrossRef]
55. Abd El-Hafeez AA, Marzouk HMM, Abdelhamid MAA, Khalifa HO, Hasanin THA, Habib AGK, et al. Anti-cancer effect of Hyoscyamus muticus extract via its activation of Fas/FasL-ASK1-p38 pathway. Biotechnol Bioprocess Eng. 2022;27(5):807-19.[CrossRef]
56. Elkhouly HI, Hamed AA, El Hosainy AM, Ghareeb MA, Sidkey NM. Bioactive secondary metabolite from endophytic Aspergillus tubenginses ASH4 isolated from Hyoscyamus muticus: Antimicrobial, antibiofilm, antioxidant and anticancer activity. Pharmacogn J. 2021;13(2):434-42.[CrossRef]
57. Belguidoum M, Dendougui H, Kendour Z.In vitro antioxidant properties and phenolic contents of Zygophyllum album L. from Algeria. J Chem Pharm Res. 2015;7(1):510-4.
58. Bourgou S, Megdiche W, Ksouri R. The halophytic genus Zygophyllum gaetulum and Nitrariafrom North Africa: A phytochemical and pharmacological overview. Med Aromat Plants World Africa. 2017;3:345-56.[CrossRef]
59. Azab A. Amaranthaceae plants of israel and palestine: Medicinal activities and unique compounds. Eur Chem Bull. 2020;9(12):366-400.[CrossRef]
60. Adegbola PI, Adetutu A, Olaniyi TD. Antioxidant activity of Amaranthusspecies from the Amaranthaceae family-A review. South African J Bot. 2020;133:111-7.[CrossRef]
61. Moulambi-Nzonza SJ, Mankessi F, Bassiloua JB, Matondo R. Effet du substrat sur la biomasse et la fixation symbiotique de l'azote par les plants de Acacia mangium willd.(Fabaceae) en pépinière. Int J Biol Chem Sci. 2020;14(5):1618-32.[CrossRef]
62. Boutoub O, Ghadraoui LE, Miguel MG. Biological properties of latex, aqueous extracts and bee products of Euphorbia officinarum L.:A short review. Molecules. 2022;27(21):7200.[CrossRef]
63. Chamkhi I, Hnini M, Aurag J. Conventional medicinal uses, phytoconstituents, and biological activities of Euphorbia officinarum L.: A systematic review. Adv Pharmacol Pharm Sci. 2022;2022:9971085.[CrossRef]
64. Jaradat NA, Al-Ramahi R, Zaid AN, Ayesh OI, Eid AM. Ethnopharmacological survey of herbal remedies used for treatment of various types of cancer and their methods of preparations in the West Bank-Palestine. BMC Complement Altern Med. 2016;16:93.[CrossRef]
65. Domazetovic V, Marcucci G, Iantomasi T, Brandi ML, Vincenzini MT. Oxidative stress in bone remodeling:Role of antioxidants. Clin Cases Miner Bone Metab. 2017;14(2):209-16.[CrossRef]
66. Coria-Téllez AV, Montalvo-Gónzalez E, Yahia EM, Obledo-Vázquez EN. Annona muricata: A comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arab J Chem. 2018;11(5):662-91.[CrossRef]
67. Naghibi F, Mosaddegh M, Motamed SM, Ghorbani A. Labiatae family in folk medicine in Iran: From ethnobotany to pharmacology. Iran J Pharm Res. 2022;4(2):63-79.
68. Gao Y, Dong Y, Guo Q, Wang H, Feng M, Yan Z,et al. Study on supramolecules in traditional chinese medicine decoction. Molecules. 2022;27(10):3268.[CrossRef]
69. Zhao J, Tian S, Lu D, Yang J, Zeng H, Zhang F, et al. Systems pharmacological study illustrates the immune regulation, anti-infection, anti-inflammation, and multi-organ protection mechanism of Qing-Fei-Pai-Du decoction in the treatment of COVID-19. Phytomedicine. 2021;85:153315.[CrossRef]
70. Wan F, Feng C, Luo K, Cui W, Xia Z, Cheng A. Effect of steam explosion on phenolics and antioxidant activity in plants: A review. Trends Food Sci Technol. 2022;124:13-24.[CrossRef]
71. Maleki SJ, Crespo JF, Cabanillas B. Anti-inflammatory effects of flavonoids. Food Chem. 2019;299:125124.[CrossRef]
72. Hadjadj S, Esnault MA, Berardocco S, Guyot S, Bouchereau A, Ghouini F, et al. Polyphenol composition and antioxidant activity of Searsia tripartita and Limoniastrum guyonianum growing in Southeastern Algeria. Sci Afr. 2020;10:00585.[CrossRef]
73. Jallali I, Zaouali Y, Mkadmini K, Smaoui A, Abdelly C, Ksouri R. Phytochemistry and antioxidant activities of Rhus tripartitum (Ucria) Grande leaf and fruit phenolics, essential oils, and fatty acids. Nat Prod Commun. 2022;17(4):1-8.[CrossRef]
74. Belhi Z, Cheriti A. Bioguided Phytochemical Study of Extracts from Some Saharan Plants and their Effect on Fusarium oxysporum f. . albedinis Cellulase Enzymes Etude Phytochimique Bioguidée des Extraits de Quelques Plantes Sahariennes et Leur Effet sur les Cellulases du Fus. Algeria:Kasdi Merbah University-Ouargla;2021.
75. Bahramsoltani R, Kalkhorani M, Zaidi SMA, Farzaei MH, Rahimi R. The genus Tamarix:Traditional uses, phytochemistry, and pharmacology. J Ethnopharmacol. 2020;246:112-245.[CrossRef]
76. Guetat A. The Genus Deverra DC. (Syn. Pituranthos Viv.):A natural valuable source of bioactive phytochemicals:A review of traditional uses, phytochemistry and pharmacological properties. J Ethnopharmacol. 2022;284:114447.[CrossRef]
77. Bendjedou H, Benamar H, Bennaceur M, Rodrigues MJ, Pereira CG, Trentin R, et al. New insights into the phytochemical profile and biological properties of Lycium intricatum Bois. (Solanaceae). Plants. 2023;12:996.[CrossRef]
78. Mohammed AESI. Phytoconstituents and the study of antioxidant, antimalarial and antimicrobial activities of Rhus tripartita growing in Egypt. J Pharmacogn Phytochem. 2015;4(2):276-81.
79. Itidel C, Chokri M, Mohamed B, Yosr Z. Antioxidant activity, total phenolic and flavonoid content variation among Tunisian natural populations of Rhus tripartita (Ucria) Grande and Rhus pentaphylla Desf. Ind Crop Prod. 2013;51:171-7.[CrossRef]
80. Ben MH, Saada M, Jallali I, Ben BZ, Tlili M, Alimi H, et al. Variability of antioxidant and biological activities of Rhus tripartitum related to phenolic compounds. Excli J. 2017;16:439-47.
81. Abcha I, Criado P, Salmieri S, Najjaa H, Isoda H, Neffati M, et al. Edible Rhus tripartitafruit as source of health-promoting compounds:Characterization of bioactive components and antioxidant properties. Eur Food Res Technol. 2019;245:2641-54.[CrossRef]
82. Karoune S, Kechebar MSA, Djellouli A, Belhamra M, Rahmoune C, Ksouri R. Variability of antioxidant properties and identification of phenolic contents by HPLC-DAD in different organs of Acacia albida and Acacia raddiana. Int J Pharmacogn Phytochem Res. 2015;8(5):701-9.
83. Ahmad S, AbdEl-Salam NM, Ullah R. In vitro antimicrobial bioassays, DPPH radical scavenging activity, and FTIR spectroscopy analysis of Heliotropium bacciferum. Biomed Res Int. 2016;2016:3818945.[CrossRef]
84. El Yahyaoui O, Ouaaziz NAIT, Guinda I, Sammama A, Kerrouri S, Bouabid B, et al. Phytochemical screening and thin layer chromatography of two medicinal plants:Adansonia digitata (Bombacaceae) and Acacia raddiana (Fabaceae). J Pharmacogn Phytochem. 2017;6(1):10-5.
85. Ziani BEC, Carocho M, Abreu RMV, Bachari K, Alves MJ, Calhelha RC, et al. Phenolic profiling, biological activities and in silico studies of Acacia tortilis (Forssk.) Hayne ssp. raddianaextracts. Food Biosci. 2020;36:100616.[CrossRef]
86. Khalaf OM, Ghareeb MA, Saad AM, Madkour HMF, El-Ziaty AK, Abdel-Aziz MS. Phenolic constituents, antimicrobial, antioxidant, and anticancer activities of ethyl acetate and n-butanol extracts of Senna italicaa. Acta Chromatogr. 2019;31(2):138-45.[CrossRef]
87. Mncube SC, Gololo SS, Mogale MA. Seasonal variations of phytochemical content and antioxidant activity of Senna italicaa leaves. Asian J Chem. 2020;32(9):2371-4.[CrossRef]
88. Adjou ES, Koudoro AY, Nonviho G, Ahoussi ED, Sohounhloue DCK. Phytochemical profile and potential pharmacological properties of leaves extract of Senna italicaaMill. Am J Pharmacol Sci. 2021;9(1):36-9.
89. Dabai YU, Kawo AH, Aliyu RM. Phytochemical screening and antibacterial activity of the leaf and root extracts of Senna italicaa. Afr J Pharm Pharmacol. 2012;6(12):914-8.[CrossRef]
90. Elaloui M, Ennajah A, Ghazghazi H, Ben YI, Ben ON, Hajlaoui MR, et al. Quantification of total phenols, flavonoides and tannins from Ziziphus jujuba (mill.) Ziziphus lotus (L.)(Desf). Leaf extracts and their effects on antioxidant and antibacterial activities. Int J Second Metab. 2017;4(1):18-26.[CrossRef]
91. Rocchetti G, Alcántara C, Bäuerl C, García-Pérez JV, Lorenzo JM, Lucini L, et al. Bacterial growth and biological properties of Cymbopogon schoenanthus and Ziziphus lotus are modulated by extraction conditions. Food Res Int. 2020;136:109534.[CrossRef]
92. Donno D, Beccaro GL, Mellano MG, Cerutti AK, Bounous G. Goji berry fruit (Lycium spp.):Antioxidant compound fingerprint and bioactivity evaluation. J Funct Foods. 2015;18:1070-85.[CrossRef]
93. Boulila A, Mattoussi K, M'rabet Y, Rokbeni N, Dhouioui M, Boussaid M. Determination of phytochemicals and antioxidant activity of methanol extracts obtained from the fruit and leaves of Tunisian Lycium intricatum Boiss. Food Chem. 2015;174:577-84.[CrossRef]
94. Kennouche S, Bentamene A. Etude Phytochimique et Biologique des Espèces Chrysanthemum segetum L. (Asteraceae) et Limonium pruinosum (L.) Chaz. (Plumbaginaceae). Algeria:UniversitéFrères Mentouri-Constantine;2017.
95. Benamira FZ, Djabar H. Évaluation de L'activitéAntibactérienne et Antifongique de Deux Plantes Algériennes:Limonium thouinii(Viv.) O. Kuntze et Capnophyllum peregrenum. Algeria:Universitédes Frères Mentouri Constantine;2021.
96. Talele CD, Patil SV. Phytochemical screeening of Launaea pinnatifitida leaves extract. Int J Pharmacol Biol Sci. 2017;11(2):21.
97. Moghal MMR, Millat MS, Hussain MS, Islam MR. Thrombolytic and membrane stabilizing activities of Launaea sarmentosa. Int J Pharmacogn. 2016;3(8):354-8.
98. Megdiche Ksouri W, Chaouachi F, M'Rabet R, Medini F, Zaouali Y, Trabelsi N, et al. Antioxidant and antimicrobial properties of Frankenia thymifolia Desf. fractions and their related biomolecules identification by gas chromatography/mass spectrometry (GC/MS) and high performance liquid chromatography (HPLC). J Med Plants Res. 2011;5(24):5754-65.
99. Carro RT, D'almeida RE, Isla MI, Alberto MR. Antioxidant and anti-inflammatory activities of Frankenia triandra (J. Rémy) extracts. South African J Bot. 2016;104:208-14.[CrossRef]
100. Mennai I, Hanfer M, Esseid C, Benayache S, Ameddah S, Menad A, et al. Chemical composition, in vitro antiparasitic, antimicrobial and antioxidant activities of Frankenia thymifolia Desf. Nat Prod Res. 2020;34(23):3363-8.[CrossRef]
101. Nagda V, Gajbhiye A, Kumar D. Isolation and characterization of endophytic fungi from Calotropis procera for their antioxidant activity. Asian J Pharm Clin Res. 2017;10(3):254-8.[CrossRef]
102. Ahmed W, Azmat R, Khan SM, Khan MS, Qayyum A, Mehmood A, et al. Pharmacological studies of Adhatoda vasica and Calotropis procera as resource of bio-active compounds for various diseases. Pak J Pharm Sci. 2018;31(5):1975-83.
103. Tsala DE, Nga N, Thiery BNM, Bienvenue MT, Theophile D. Evaluation of the antioxidant activity and the healing action of the ethanol extract of Calotropis procerabark against surgical wounds. J Intercult Ethnopharmacol. 2015;4(1):64-9.[CrossRef]
104. Alghanem SM, El-Amier YA. Phytochemical and biological evaluation of Pergularia tomentosa L. (Solanaceae) naturally growing in arid ecosystem. Int J Plant Sci Ecol. 2017;3(2):7-15.
105. Arshad MA, Ahmad S, Khurshid U, Pervaiz I. Studies on the antioxidant and xanthine oxidase inhibition potential of Heliotropium crispum. Acta Pol Pharm. 2018;75(1):41-4.
106. Aïssaoui H, Mencherini T, Esposito T, De Tommasi N, Gazzerro P, Benayache S, et al. Heliotropium bacciferum Forssk. (Boraginaceae) extracts:Chemical constituents, antioxidant activity and cytotoxic effect in human cancer cell lines. Nat Prod Res. 2019;33(12):1813-8.[CrossRef]
107. Pothiraj C, Balaji P, Shanthi R, Gobinath M, Babu RS, Munirah AAD, et al. Evaluating antimicrobial activities of Acanthus ilicifoliusL. and Heliotropium curassavicum L against bacterial pathogens:An in-vitro study. J Infect Public Health. 2021;14(12):1927-34.[CrossRef]
108. Odeh AA, Al-Jaber HI, Barhoumi LM, Shakya AK, Al-Qudah MA, Al-Sanabra OM. Phytochemical and bioactivity evaluation of secondary metabolites and essential oils of Sedum rubens growing wild in Jordan. Arab J Chem. 2023;16(6):104712.[CrossRef]
109. Söhretoglu D, Gença Y, Harput ÜS, Sabuncuoglub S, Šoralc M, Rendad G, et al. Phytochemical content, antioxidant and cytotoxic activities of Sedum spurium. Nat Prod Commun. 2016;11(11): 1693-6.
110. Uduak A, Hadiza K. Qualitative and quantitative phytochemical analysis of Maerua crassifolia leaves using various solvents. Niger J Pure Appl Sci. 2019;32(1):3315-23.
111. Yonbawi AR, Abdallah HM, Alkhilaiwi FA, Koshak AE, Heard CM. Anti-proliferative, cytotoxic and antioxidant properties of the methanolic extracts of five Saudi Arabian flora with folkloric medicinal use:Aizoon canariense, Citrullus colocynthis, Maerua crassifolia, Rhazya stricta and Tribulus macropterus. Plants. 2021;10(10):2073.[CrossRef]
112. Mounira GM, Nouha K, Ons D, Mohamed N, Romdhane M. Variability in the chemical composition and phenolic compounds of wild Tunisian populations of Nitraria retusa (Forssk.) Asch. and study of their antioxidant properties. AFRICAN J Biotechnol. 2023;22(4):71-9.[CrossRef]
113. Kumar Vaidya S, Bothra SB, Santosh A, Vaidya K. An ethno-phytochemical and pharmacological review on some unexplored medicinal plants belongs to north-east and south-east region of Chattishgarh. Eur J Pharm Med Res. 2014;1(1):240-61.
114. Tahrouch S, Rapior S, Belahsen Y, Bessière JM, Andary C. Volatile constituents of Peganum harmala(Zygophyllaceae). Acta Bot Gall. 1998;145(2):121-4.
115. Kchaou M, Ben SH, Mhiri R, Allouche N. Anti-oxidant and anti-acetylcholinesterase activities of Zygophyllum album. Bangladesh J Pharmacol. 2016;11(1):54-62.[CrossRef]
116. Zheng Y, Luo F, Wang L, Li S, Wang X, Zhu Z. Comparison of the characteristics of phenolic compounds in Se-enriched kiwifruit and conventional kiwifruit. Food Chem X. 2025;27:102453.[CrossRef]
117. KnapováP, BilkováA, Vávra R. Total phenolic content and antioxidant activity of apple cultivars. Acta Hortic. 2021;1329:15-9.[CrossRef]
118. Erdogan MK, Toy Y, Gundogdu R, Gecibesler IH, Sever A, Yapar Y, et al. Assessment of cytotoxic, apoptotic, enzyme inhibitory, and antioxidant properties, and phytochemical characterization of ethanolic extract from Cionura erecta. Food Biosci. 2025;65:106082.[CrossRef]
119. Gecibesler IH, Behcet L, Erdogan MK, Askin H. Antioxidant potencies and chemical compositions of essential oils of two endemic species grow in Turkey:Astragalus oocephalus subsp. and Astragalus sericans. Prog Nutr. 2017;19(6):60-7.
120. Tlili H, Macovei A, Buonocore D, Lanzafame M, Najjaa H, Lombardi A, et al. The polyphenol/saponin-rich Rhus tripartita extract has an apoptotic effect on THP-1 cells through the PI3K/AKT/mTOR signaling pathway. BMC Complement Med Ther. 2021;21(1):153.[CrossRef]
121. Zibaee E, Akaberi M, Tayarani-Najaran Z, Nesmerák K, Štícha M, Shahraki N, et al. Comparative LC-ESIMS-based metabolite profiling of Senna italicaa with Senna alexandrina and evaluating their hepatotoxicity. Metabolites. 2023;13(4):559.[CrossRef]